Curated OER
2000 U.S. National Chemistry Olympiad National Exam - Part I
The National Chemistry Olympiad exams are comprehensive tests covering an entire year of chemistry concepts. You can use them as practice for competing in the challenge, or simply as a review, or as an actual final exam for your general...
Curated OER
2009 U.S. National Chemistry Olympiad National Exam - Part I
The 2009 version of the first part of a national chemistry competition is posted for your use with olympiad hopefuls. Test takers deal with 60 multiple choice questions covering an entire year of chemistry curriculum. Use this to...
Utah Education Network (UEN)
Utah Open Textbook: Chemistry
Technology can help save money and add convenience. The resource offers a free textbook for a complete Chemistry course. The text begins with a review of the scientific method and continues to explain topics such as chemical bonding,...
Cornell University
Atomic Bonding
Explore the connection of surface area to bonding within atoms. Learners complete lab investigations to model changing surface area with different sizes and concentrations of atoms. A flour fireball demonstration follows the labs to...
Chemistry Collective
Virtual Lab: ATP Reaction (Thermochemistry and Bonding)
If you've ever felt a little drained ... try converting some ATP to ADP! Science scholars perform one of life's most important reactions using a simulated lab workbench. The fun-to-use interactive allows pupils to control the reactants...
Chemistry Collective
Inelastic Collisions
That's the way the ball bounces ... if the bonds cooperate! Young scholars use a simulation experiment to explore the bonds between atoms in bouncing balls. They adjust the bond strength of two balls to compare the reactions after the...
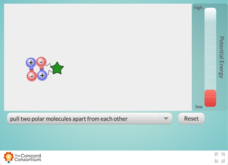
Concord Consortium
Comparing Potential Energy of a Bond
Have you reached your breaking point in looking for great resources that illustrate bond energy? Demonstrate the potential energy changes that occur when polar and non-polar bonds are broken with a stimulating simulation. Pupils pull on...
Concord Consortium
Energy of Bond Formation
Show your chemistry class that there's much more to covalent bonding than sharing electrons! Pupils manipulate atoms of hydrogen, oxygen, and carbon to observe the energy of bond formation using a well-rounded interactive. The resource...
Science Geek
Covalent Bonding
When it comes to covalent bonds, sharing is caring. Presentation covers the octet rule with multiple examples, Lewis Dot Structures with an example, and resonance. Presentation is the first in a five-part series.
Curated OER
Estimating Heat Changes during Reactions Using Bond Energies
For advanced chemists, this instructional activity examines heat change during chemcial reactions. Alkanes are examined first. Learners calculate the energy released or needed during a reaction.
Curated OER
Vocabulary - Unit 5, Chemical Bonds
All you will find in this resource is a list of vocabulary terms dealing with chemical bonds. Space is provided for chemistry learners to write out definitions. This would be a terrific tool to help prepare them for a quiz.
Curated OER
Electromagnetic Spectrum
In this electromagnetic spectrum worksheet, students review all regions of the spectrum in order from longest to shortest wavelength. Students list all possible values for specific quantum numbers. This worksheet has 20 problems to solve.
Curated OER
A Potpourri of Thermo Questions
In this thermodynamics instructional activity, learners match terms to their definitions such as enthalpy, equilibrium, bond energy, and heat capacity. This instructional activity has 20 words to match.