Simon Fraser University
Chem1 Virtual Textbook: What Are Line Spectra?
Acting as part of an overview on quantum theory, this section of the site answers the question, what are line spectra? In addition to the definition and a discussion of related terms, the site also discusses how line spectra are organized.
CK-12 Foundation
Ck 12: Atomic Emission Spectra
[Free Registration/Login may be required to access all resource tools.] In this lesson, students learn about atomic emission spectra. Includes a simulation for exploring the Blackbody Spectrum.
University of Colorado
University of Colorado: Physics 2000: Spectral Lines
Several pages from an excellent site which describe the science of spectroscopy. The unique atomic emission (and absorption) line spectrum of elements are illustrated and explained. Includes a Java applet depicting the quantum energy...
University of Colorado
University of Colorado: Physics 2000: Quantum Atom
Several pages with an interesting discussion of the visible light spectrum and atomic absorption and emission line spectrum. Features excellent graphics, thorough and understandable discussion, and many interactive Java applets.
Khan Academy
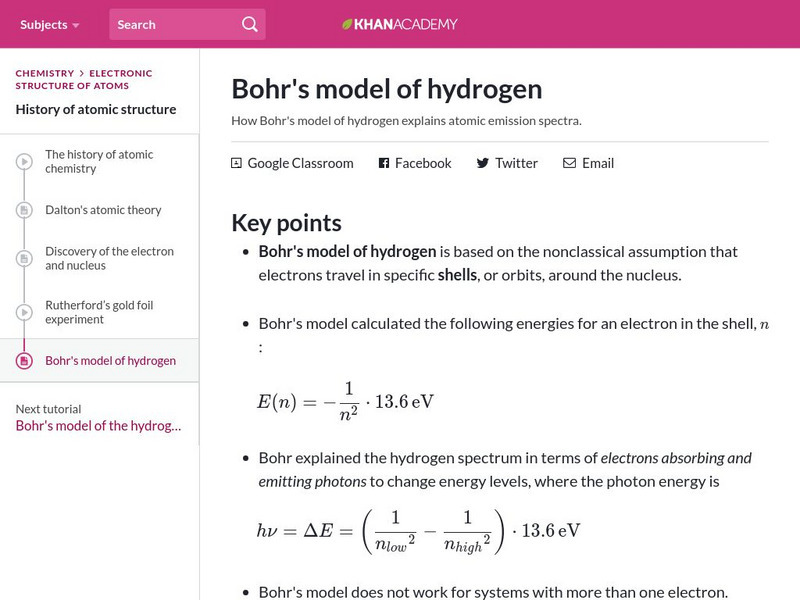
Khan Academy: Bohr's Model of Hydrogen
Resource investigates how Bohr's model of hydrogen explains atomic emission spectra.
Friesian School
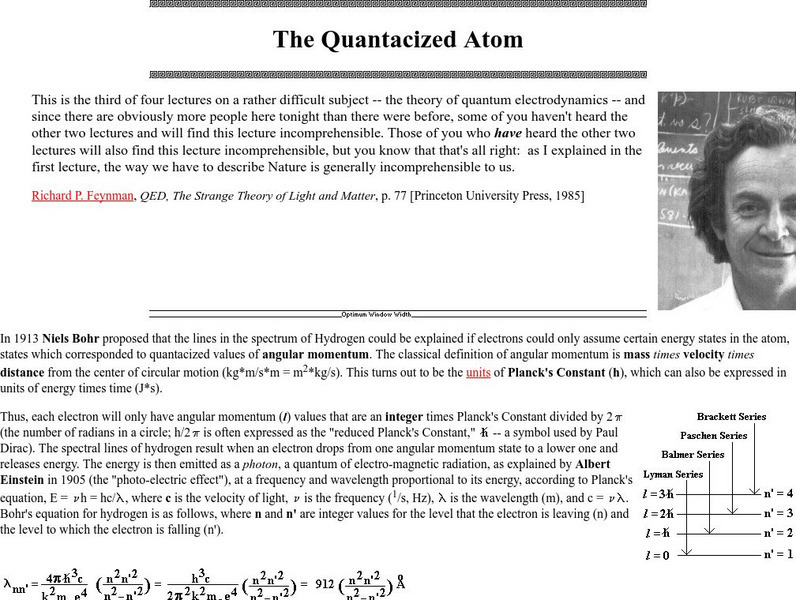
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
CK-12 Foundation
Ck 12: Plix: Atomic Emission Lamps: Atomic Emission Spectra
[Free Registration/Login Required] In this interactive you will drag each gas bulb into the red circle to excite them and see their emission spectrum. You will need a sign-in to access this media, but it will be worth your time!
State University of New York
State University of New York: Atomic Absorption and Emission
This module simulates the excitation of hydrogen atoms through irradiation with electromagnetic radiation of different wavelengths.
CK-12 Foundation
Ck 12: Light
[Free Registration/Login may be required to access all resource tools.] In the following tutorial students will describe the relationships between speed, wavelength, and frequency of light. They will understand the photoelectric effect...
Michael Blaber, PhD
Florida State University: The Bohr Model of the Atom
A well designed clear tutorial explaining the energies involved in the Bohr model of the atom. Illustrations add to the clearly presented equations.
CK-12 Foundation
Ck 12: The Bohr Model of the Atom
[Free Registration/Login may be required to access all resource tools.] Students will learn about the history of atomic theory, and the development of the Bohr model of the atom. Includes a simulation for exploring the Bohr Model.
Khan Academy
Khan Academy: Bohr's Model of Hydrogen
How Bohr's model of hydrogen explains atomic emission spectra.
Dartmouth College
Dartmouth College: Chem Lab: Welcome to General Chemistry Laboratory!
Geared towards helping Dartmouth College students starting General Chemistry Laboratory, this site offers resources important for anyone in the lab. Learn why you should keep a lab notebook and how to keep yourself and others safe. This...
Wikimedia
Wikipedia: Henry Moseley
This site from the Wikipedia Encyclopedia provides an overview of the life of scientist Henry Moseley. This open-source encyclopedia site discusses his contribution to science and how he found a systematic relation between wavelength and...
Nobel Media AB
The Nobel Prize: Niels Bohr Biographical
The Nobel Foundation provides this site about Niels Bohr's contributions to the world of physics, specifically his "investigation of the structure of atoms and of the radiation emanating from them." This biography includes information on...
CK-12 Foundation
Ck 12: Chemistry Simulation: Neon Lights
[Free Registration/Login Required] Neon lights are a type of discharge tube. Observe how electrons create colored light in a hydrogen gas discharge tube. Can you figure out why hydrogen's emission spectrum contains more than one color of...
CK-12 Foundation
Ck 12: The Bohr and Quantum Models of the Atom
[Free Registration/Login may be required to access all resource tools.] Students explore how the study of the hydrogen emission spectrum led to the Bohr model of the atom, in which electrons exist in states of constant energy.
Cosmo Learning
Cosmo Learning: Chemistry 1 A: General Chemistry
A collection of video lectures from a general chemistry course taught at the University of California, Berkeley. The course covers topics like stoichiometry, acid-base and solubility equilibrium, oxidation-reduction reactions, chemical...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...