Science Education Resource Center at Carleton College
Serc: Rutherford's Enlarged: A Content Embedded Activity on Nature of Science
Through a study of Ernest Rutherford's experiments with atomic structure, students develop a deeper understanding of the nature of science. The activity is taken from a linked article by the author that was published in the Journal of...
Texas Instruments
Texas Instruments: Atomic Structure
This StudyCards stack enables students to review the terms associated with learning and using the Periodic Table of the Elements.
Science Education Resource Center at Carleton College
Serc: Quantum Atomic Structure
This multi-day lesson plan helps students to understand how the models of the atom have changed and how quantum mechanics affects the electrons as they orbit the nucleus.
PBS
Pbs Learning Media: The Atom
In this interactive activity from ChemThink, students will take a closer look at atomic structure, properties, and behaviors. Includes background reading material and discussion questions.
Concord Consortium
Concord Consortium: What Are Nature's Building Blocks?
Activity 4 What are the electrons? This question is answered in the context of Niels Bohr's model and the probability model of atomic structure.
Texas Instruments
Texas Instruments: Atomic Nuclear
This StudyCards stack enables students to review the vocabulary associated with atoms and nuclear chemistry.
Annenberg Foundation
Annenberg Learner: Interactives: Periodic Table
Learn the basics of the Periodic Table including how the Periodic Table is organized and how to make sense of the information included in the table. Test your understanding with an interactive quiz at the end of the activity.
Concord Consortium
Concord Consortium What Are Nature's Building Blocks?
The module will develop, revise, and interpret representations of atomic structure and interactions of charges within atoms. The following activities will be explored: Activity 1. What are the particles that make up all substances and...
Concord Consortium
Concord Consortium: What Makes Water Special?
In Activity 1 investigates How are water and other liquids similar and different? The student will see if ideas involving energy, electrostatic interactions, and atomic structure, can be used to explain why a hurricane is so powerful.
Science Education Resource Center at Carleton College
Serc: Tic Tac Toe Pick 3 in a Row Atomic Model Assignment
A choice menu assignment where students select between several extension activities that help understand the structure of the atom.
Concord Consortium
Concord Consortium: Science of Atoms and Molecules: Nucleic Acids and Proteins
Through this activity, students work with macromolecules, proteins and nucleic acids. The focus is on the atomic structure of proteins, how linear polymers are made, and the surface charges of the resulting polymers. . Multiple-choice...
Concord Consortium
Concord Consortium: What Is Happening When a Spark Occurs?
Students define potential and kinetic energy, explore energy transfer and energy conservation, and connect energy to atomic structure. The following activities guide students to a conclusion: Activity 1. Can my finger start a fire?...
PBS
Nova: The Atom Builder
A brief explanation is provided for designing a stable atom. You can also refer to a labeled model of a carbon atom. This resource also has a link to an atom building activity.
Nobel Media AB
The Nobel Prize: William Lawrence Bragg Biographical
The official Nobel biography of William Lawrence Bragg. the son of William Henry Bragg, who shared the 1915 Nobel Prize in Physics with his father. This article focuses on Bragg's science activities, positions, and achievements and honors.
PBS
Nova: Atom Builder
Find out if you know enough about atoms to build them. The goal of the activity is to build an atom of a particular element by dragging the correct numbers of neutrons, protons and electrons into the atom.
PBS
Pbs Learning Media: That's My Theory!
Become a game show contestant in this online activity from A Science Odyssey and ask a series of questions to a panel of mystery scientists, using the answers to determine which scientist is Einstein.
CK-12 Foundation
Ck 12: Orbitals
[Free Registration/Login may be required to access all resource tools.] A collection of learning opportunities about electron orbitals. Includes videos, activities, discussion questions, and quizzes.
Concord Consortium
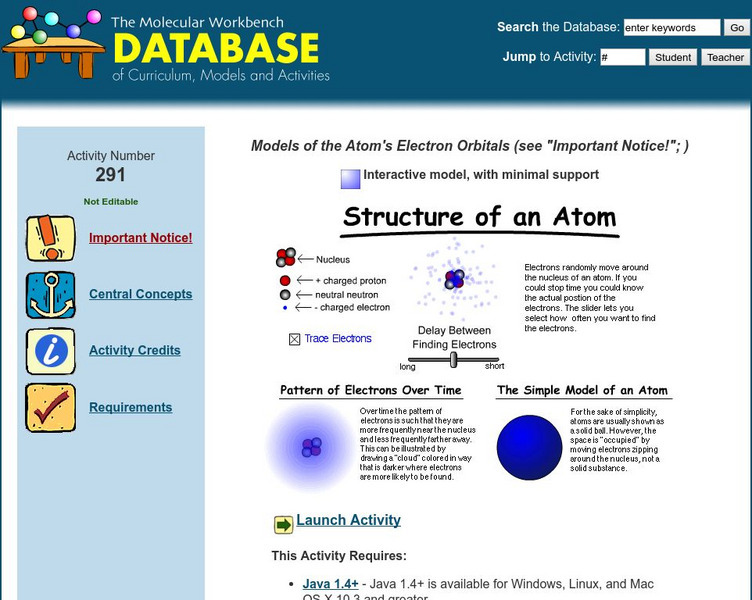
The Molecular Workbench Database: Models of the Atom's Electron Orbitals
Learn about atomic structure and the multiple theories of atomic structure in this simulation.
Texas Instruments
Texas Instruments: The History Behind the Atom
This StudyCard activity enables students to review the key contributions by scientists and philosophers towards are current understanding of the atom. It also allows students to review characteristics of the major atomic models.
Science Education Resource Center at Carleton College
Serc: Electron Energy Levels of Atoms and Ions
In this lab, students investigate basic electron structure by making a model using pennies and different sized filter papers.
Concord Consortium
Concord Consortium: Where Does All the Energy in an Explosion Come From?
In this module Activity 2 investigates What happens to atoms during a chemical reaction? In this activity students explore chemical reactions and develop a model to explain observations of chemical reactions.
Concord Consortium
Concord Consortium: How Can a Small Spark Start a Huge Explosion?
In this module Activity 4 investigates How are bonds formed and broken? This activity explores the relationship between energy and the formation and breaking bonds, using the ideas of energy transfer and conversion.
Concord Consortium
Concord Consortium: How Can a Small Spark Start a Huge Explosion?
In this module Activity 3 investigates When atoms get close to each other, what happens to their potential energy? This activity applies energy concepts to compare the potential energy of atoms that are bonded as molecules with the...
Science Education Resource Center at Carleton College
Serc: Drawing Atoms
This activity serves as an introduction to chemistry, and can be used to help students draw a two dimensional image of the atom.