Michael Blaber, PhD
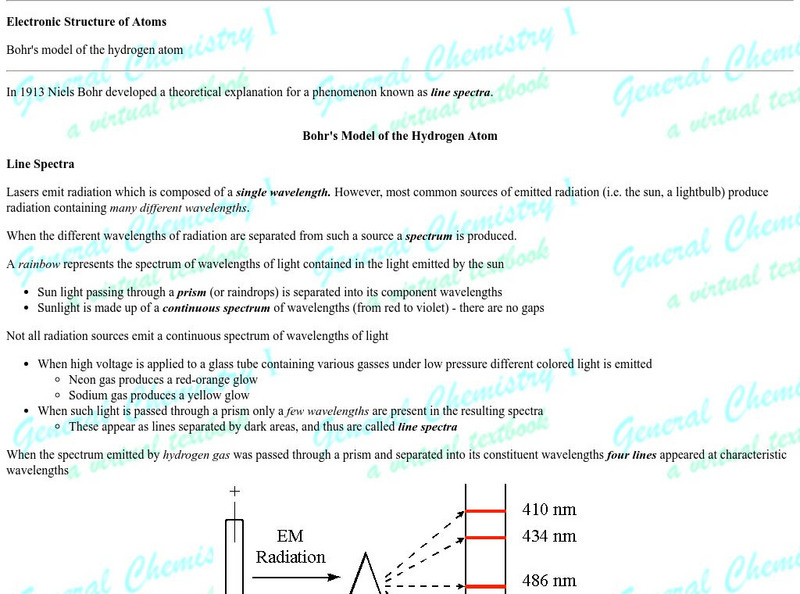
Florida State University: The Bohr Model of the Atom
A well designed clear tutorial explaining the energies involved in the Bohr model of the atom. Illustrations add to the clearly presented equations.
CK-12 Foundation
Ck 12: The Bohr Model of the Atom
[Free Registration/Login may be required to access all resource tools.] Students will learn about the history of atomic theory, and the development of the Bohr model of the atom. Includes a simulation for exploring the Bohr Model.
Khan Academy
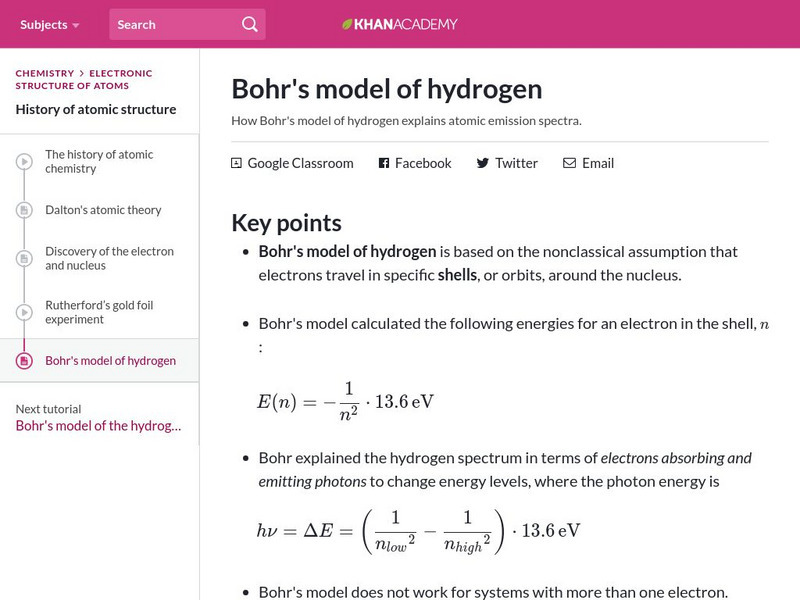
Khan Academy: Bohr's Model of Hydrogen
Resource investigates how Bohr's model of hydrogen explains atomic emission spectra.
Khan Academy
Khan Academy: Bohr's Model of Hydrogen
How Bohr's model of hydrogen explains atomic emission spectra.
Other
Siyavula Education: Everything Maths & Science: Models of the Atom
Discusses models of the atom developed by John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick. Includes clear illustrations and a short comprehension exercise at the end.
CK-12 Foundation
Ck 12: The Bohr and Quantum Models of the Atom
[Free Registration/Login may be required to access all resource tools.] Students explore how the study of the hydrogen emission spectrum led to the Bohr model of the atom, in which electrons exist in states of constant energy.
Other
Cronodon: Atoms Models of the Atom
Presents models of the atom developed by Thomson, Rutherford, Bohr, and Schrodinger. Includes successes and failures of each, illustrations, and detailed descriptions. Also discusses Dirac's model of the atom and introduces quantum...
Boise State University
Boise State University: Atoms: A Virtual Field Trip Through Time and Space
Learn about the models of the atom that have been proposed throughout history. Presents Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and the modern theory of the atom. Sections are accompanied by journal...
Sophia Learning
Sophia: Models of the Atom: Lesson 3
Describe how the model of the atom advanced as scientific knowledge increased. This lesson is 3 of 3 in the series titled "Models of the Atom."
Simon Fraser University
Chem1 Virtual Textbook: Atoms and the Periodic Table
As part of the General Chemistry Virtual Textbook, this site examines a list of related topics (in hyperlink format) on the atom and the Periodic Table of elements. Topics from light, particles, and waves to the Bohr model, quantum...
Science Struck
Science Struck: The Structure of an Atom: A Labeled Diagram
Looks at the scientists who developed the model of the atom by building on previous scientists' discoveries about its structure.
Other
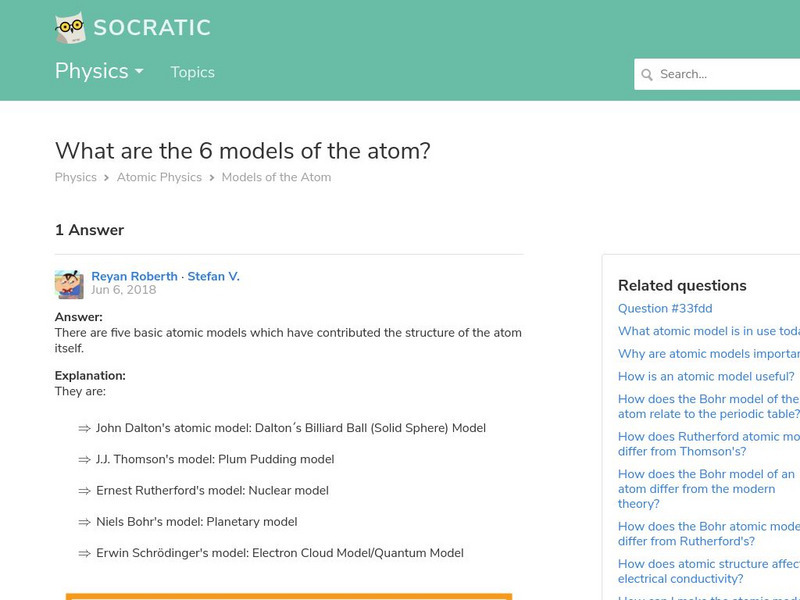
Socratic: What Are the 6 Models of the Atom?
Brief explanation of six major models of the atom along with illustrations. Covers the Greek model (Democritus), John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and the modern electron cloud or quantum mechanics model.
Other
Erik's Chemistry Page: Electronic Structure of Atoms
A description of quantum theory, the Bohr model of the atom, the quantum mechanical atom, the Scrodinger equation, and quantum numbers.
Texas Education Agency
Texas Gateway: Atoms, Elements and the Periodic Table: The Atomic Model [Pdf]
A slideshow looking at the contributions of scientists over time to our understanding of atomic theory. Looks at models of the atom developed by Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick, as...
Simon Fraser University
Chem1 Virtual Textbook: Bohr's Model
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses Niels Bohr and his work with the atom. This part of the discussion, focusing on the model itself, brings up...
Tom Richey
Slide Share: Atomic Models: Everything You Need to Know
A detailed slideshow for Grade 8 students that looks at the history of our understanding of atoms. Looks at the ideas presented by Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr, and the modern-day wave model. A...
Other
Crocodile Clips: Absorb Chemistry: History of the Atom
A tutorial that presents models of the atom proposed by John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr. Each is supported by an animated illustration. Includes comprehension questions and a quiz at the end.
Sophia Learning
Sophia: Models of the Atom: Lesson 2
Describe how the model of the atom advanced as scientific knowledge increased. This lesson is 2 of 3 in the series titled "Models of the Atom."
Sophia Learning
Sophia: Models of the Atom: Lesson 1
Describe how the model of the atom advanced as scientific knowledge increased. This lesson is 1 of 3 in the series titled "Models of the Atom."
University of Colorado
University of Colorado: Physics 2000: Bohr's Atom
An outstanding site that can best be described as student friendly. It describes the main differences between the classical and quantum models of the atom. Clear and well illustrated.
Simon Fraser University
Chem1 Virtual Textbook: Spectrum of the Hydrogen Atom
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses the hydrogen atom and its relation to spectrum. Included in the discussion is information on the Bohr model...
Simon Fraser University
Chem1 Virtual Textbook: The Bohr Atom
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses Niels Bohr and his work with the atom. Topics covered in the discussion include the atom before Bohr, Bohr's...
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Models of the Hydrogen Atom
How did scientists figure out the structure of atoms without looking at them? Try out different models by shooting light at the atom. Check how the prediction of the model matches the experimental results.
CK-12 Foundation
Ck 12: Modern Atomic Theory
[Free Registration/Login may be required to access all resource tools.] Rutherford's model of the atom was better than earlier models. But it wasn't the last word. Danish physicist Niels Bohr created a more accurate and useful model....