Khan Academy
Khan Academy: Single and Multiple Covalent Bonds
Explains what covalent bonds are and the difference between them and ionic bonds. Discusses the properties of polar and non-polar covalent bonds, and single and multiple covalent bonds, and gives analogies and examples of how covalent...
Texas Education Agency
Texas Gateway: Ionic and Covalent Bonding
In this tutorial, students learn about ionic and covalent bonds and predict which elements will form which type of bond. Includes interactive exercises as well as videos.
PBS
Pbs Learning Media: Covalent Bonding
This interactive activity from ChemThink takes a closer look at a covalent bond: how it is formed and how the sharing of two electrons can keep atoms together.
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Covalent Bonds
Explore tunneling splitting in double well potentials. This classic problem describes many physical systems, including covalent bonds, Josephson junctions, and two-state systems such as spin 1/2 particles and ammonia molecules. Java...
American Chemical Society
Middle School Chemistry: Energy Levels, Electrons, and Covalent Bonding
Students discover the concept that two atoms can attract and form a covalent bond.
Simon Fraser University
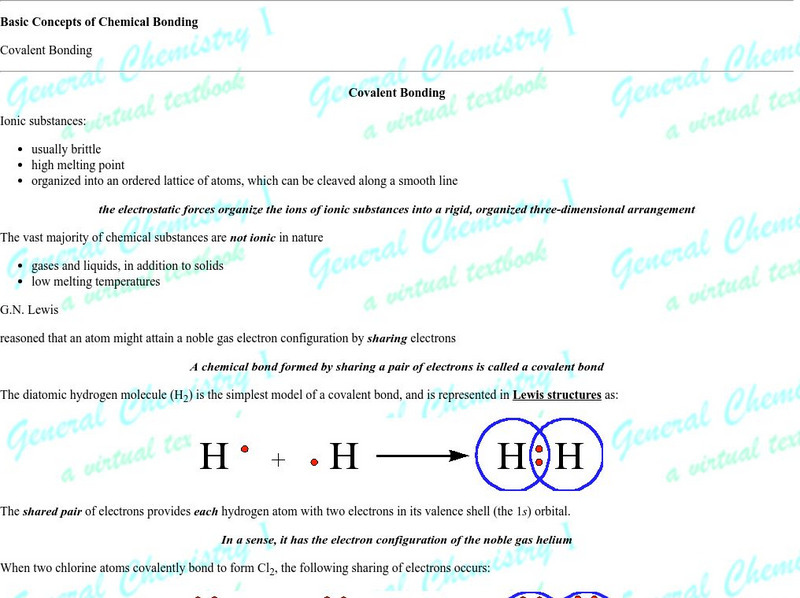
Chem1 Virtual Textbook: The Shared Electron Covalent Bond
The General Chemistry Virtual Textbook, or Chem 1, is broken into several sections covering various aspects of topics related to chemistry. This section deals with the shared-electron covalent bond and related topics such as the octet...
CK-12 Foundation
Ck 12: Physical Science: Covalent Bonding
[Free Registration/Login may be required to access all resource tools.] Definition of covalent bond, the compounds and elements they make up, and why they form.
CK-12 Foundation
Ck 12: Chemistry: Covalent Bonding
A learning module giving the definition of a covalent bond, the compounds and elements they make up and why they form. Module includes reading and review questions.
Khan Academy
Khan Academy: Covalent Bonds Questions
Practice for the MCAT with these questions on covalent bonds.
Vision Learning
Visionlearning: Chemistry: The Nature of the Chemical Bond
This site talks about ionic, polar covalent, and non-polar covalent bonding.
BBC
Bbc: Gcse Bitesize: Bonding: Covalent Bonds
This section of the lesson module focuses on covalent bonds, which are formed between non-metal atoms that share a pair of electrons. It includes links to a video and a test. Other types of chemical bonds are covered elsewhere in the...
Khan Academy
Khan Academy: Test Prep: Mcat: Chemical Processes: Covalent Bonds: Intramolecular and Intermolecular Forces
Explains what intramolecular and intermolecular forces are and the different types for each. Includes lots of examples.
Concord Consortium
Concord Consortium: Stem Resources: Chemical Bonds
By working through this web-based activity, students differentiate between ionic, non-polar covalent, and polar covalent bonds. Specifically, distinctions are made between bonding types based on orbital shapes and electronegativity...
Michael Blaber, PhD
Florida State University: Basic Concepts of Covalent Bonding: Covalent Bonding
Good introduction and graphics make this a solid page for understanding the orbital role in bonding and molecular geometry. The author is a professor at Florida State University.
Texas Education Agency
Texas Gateway: Covalent Bonding: Electron Dot Diagrams
Given descriptions, diagrams, scenarios, or chemical symbols, students will model covalent bonds using electron dot formula (Lewis structures).
American Chemical Society
Middle School Chemistry: Energy Levels, Electrons, and Covalent Bonding
Discover how covalent molecular bonding affects the energy levels of electrons.
Texas Instruments
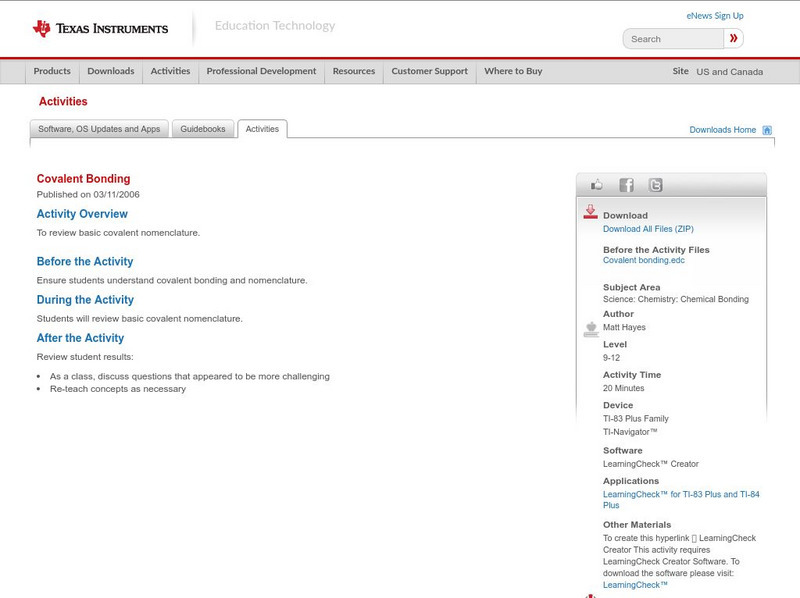
Texas Instruments: Covalent Bonding
This activity helps students review basic covalent nomenclature.
Georgia Department of Education
Ga Virtual Learning: Physical Science: Bonding and Chemical Reactions
Through informational text, interactive puzzles, and review questions, students differentiate ionic and covalent bonds and identify the properties of each. They also use oxidation numbers to predict formulas of ionic compounds, name...
CK-12 Foundation
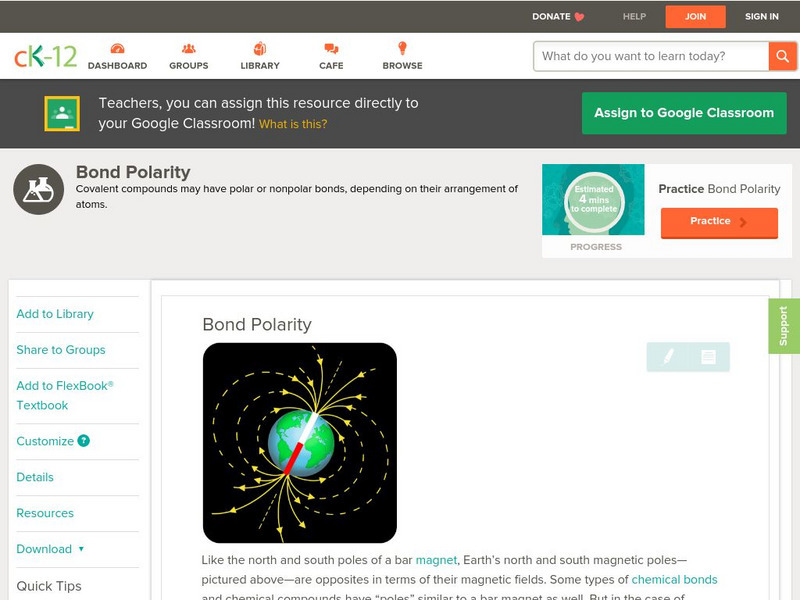
Ck 12: Physical Science: Bond Polarity
[Free Registration/Login may be required to access all resource tools.] Polarity and covalent bonds.
Other
Personal Site: Covalent Bonding Single Bonds
This site is a personal site that gives "a simple view of covalent bonding" including explanations, diagrams, and examples.
University of Florida
University of Florida: General Chemistry I: Chemical Bonding
Notes on covalent bonding, bond length, bond energies, Lewis dot structures, and percent ionic character. Colorful graphics.
McMaster University
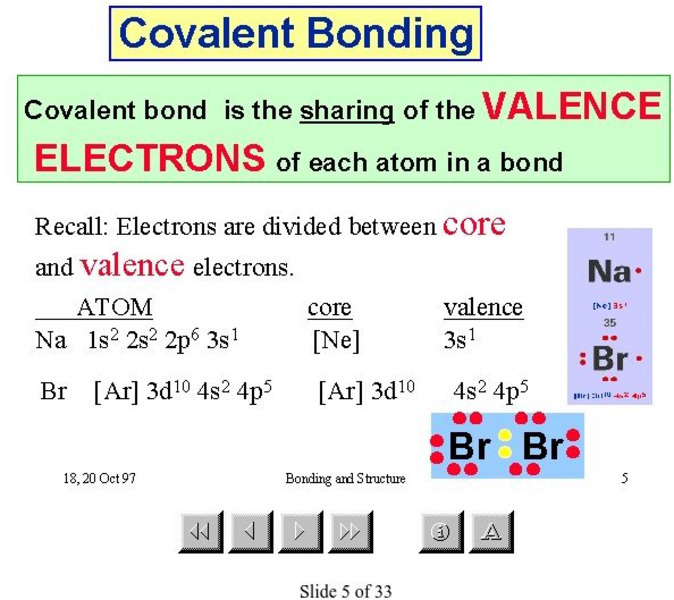
Mc Master University: Covalent Bonding
Slides 5 through 8 in this presentation from the McMaster University explain covalent bonding.
Georgia Department of Education
Ga Virtual Learning: Ap Chemistry: Bonding
In this module students explore the properties of chemicals with covalent and ionic bonds. Students learn that there are different types of covalent bonds, polar and nonpolar, and that the type of covalent bond along with the shape of a...
BBC
Bbc: Gcse Bitesize: Covalent Bonds
A covalent bond is formed between non metal atoms, which combine together by sharing electrons. Covalent compounds have no free electrons and no ions so they don't conduct electricity.
Other popular searches
- Ionic and Covalent Bonds
- Naming Covalent Bonds
- Ionic Covalent Bonds
- Ionic vs Covalent Bonds
- Polar Covalent Bonds
- Covalent Bonds in Computers
- Ionic Versus Covalent Bonds
- Covalent Bonds Beans
- Covalent Bonds Lab
- Ionic Covalent Bonds Labs
- Non Polar Covalent Bonds
- Ion and Covalent Bonds