Curated OER
Chemistry 112: Form A-
In this chemistry 112 worksheet, students use their knowledge of chemistry concepts to answer the multiple choice questions provided. Students calculate the answers using the appropriate formulas.
Curated OER
Periodic Table & Its Trends
Students review atomic structure and then participate in a activity in which they categorize several "elements" into some form of order based on their properties. They discuss the trends they see. They also practice several electron...
Curated OER
Periodic Table & Its Trends-Day 1
Students are introduced to the periodic table. They find the common trends among the electron configurations and the names of certain groups of the table. Students explore the atomic mass, atomic number, mass number, mass and charge...
Curated OER
Swinging Electrons
Pupils illustrate paramagnetic and diamagnetic differences due to the unfilled and filled valence shells. They prepare saturated solutions and place into test tubes.
Georgia State University
Georgia State University: Hyper Physics: Electron Spin
An excellent overview of electron spin, what it means in non-mathematical terms and quantum mechanical terms.
Georgia State University
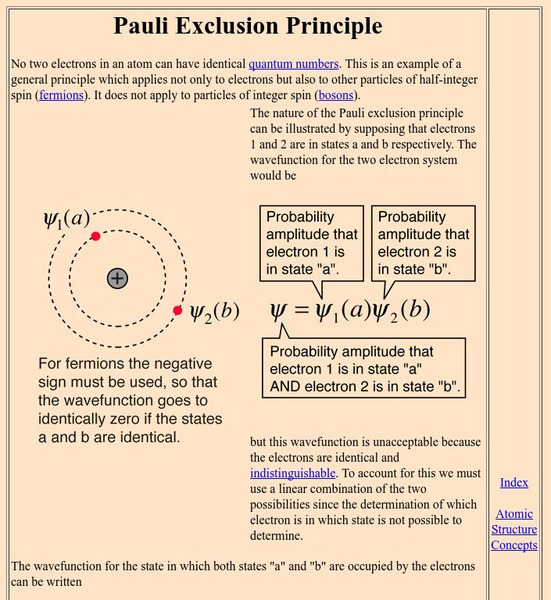
Georgia State University: Hyper Physics: Pauli Exclusion Principle
An explanation of the Pauli Exclusion Principle in mathematical terms.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: The Pauli Exclusion Principle
The Pauli Exclusion Principle shows how electrons fill atomic orbitals. Includes biographical information on Wolfgang Pauli.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: Electron Clouds and Energy Levels
An explanation of the different types of atomic orbitals, how they are filled according to the Pauli Exclusion Principle, and how many electrons can fit in each electron shell.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: Quantum Numbers
Each electron has a set of quantum numbers that specify it's location, orbital, and energy in a unique manner.
Michael Blaber, PhD
Florida State University: Electronic Structure of Atoms: Electron Configurations
Florida State University provides this article on electronic configurations of atoms and Hund's rule.
Other
Indiana University Northwest: The Aufbau Principle
The Aufbau Principle defined. Site contains charts, graphs, and examples to help in your learning of the Principle and its many purposes.
CK-12 Foundation
Ck 12: The Quantum Mechanical Model
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will calculate the wavelength, frequency, and energy of light using Planck's constant and the speed of light. They will...
CK-12 Foundation
Ck 12: Electron Arrangement in Atoms
[Free Registration/Login may be required to access all resource tools.] In the following online tutorail students will learn how to express the arrangement of electrons in atoms through electron configurations and Lewis valence electron...
CK-12 Foundation
Ck 12: Flex Book Textbooks: Chemistry Second Edition
[Free Registration/Login may be required to access all resource tools.] A complete, web-based, multi-media textbook covering a wide variety of Chemistry concepts.
Georgia Department of Education
Ga Virtual Learning: Ap Chemistry: Atomic Theory
In this module students explore how matter is classified, the history of atomic theory, subatomic particles, modern atomic theory, electron configuration, the periodic table and its trends, and spectroscopy.
Khan Academy
Khan Academy: Counting Valence Electrons
Identify the number of valence electrons of given elements.
Google
Google for Education: Model Electron Configuration Using Computational Thinking
With this demonstration, young scholars will learn to use computational thinking to better understand how the atomic number of an element affects how its electrons are configured.
Simon Fraser University
Chem1 Virtual Textbook: Atomic Electron Configurations
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses a series of topics related to electrons including atoms that have only one electron, effects of...
Vision Learning
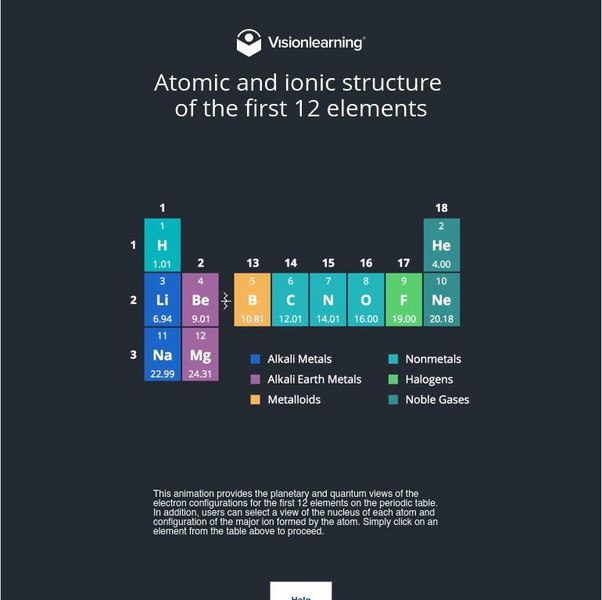
Visionlearning: Atomic and Ionic Structure of First 12 Elements
Analyze the electron configuration of the of the first twelve elements on the Periodic Table.
Other
University of Texas: Tabled Discussion
At this University of Texas site, atomic orbital occupancy, quantum numbers, the Aufbau Principle, and periodic trends are described in detail.
Vision Learning
Visionlearning: Atomic Theory and Structure: The Periodic Table
A description of the arrangement of elements on The Periodic Table based on electron configuration.
CK-12 Foundation
Ck 12: Ions
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will be able to determine the number of valence electrons for any element and draw an electron dot diagram for any atom....
Chem Tutor
Chem tutor.com: Atomic Structure
This page provides a thorough and comprehensible discussion of the structure of the atom. Includes helpful diagrams and instructions for filling electron shells, reading the Periodic Table, and drawing Lewis Structures.
CK-12 Foundation
Ck 12: Electron Configuration and the Periodic Table
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will sse the Periodic Table to identify and explain the properties of chemical families, including alkali metals, alkaline...