Hi, what do you want to do?
Khan Academy
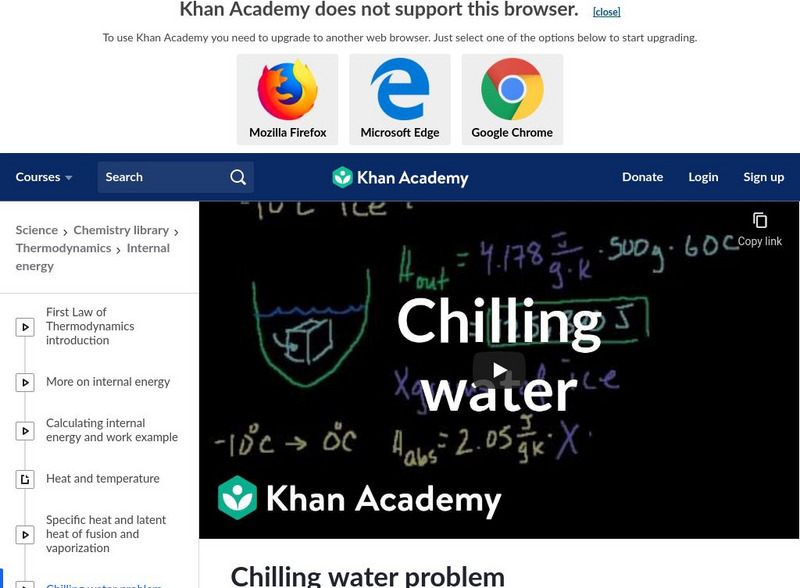
Khan Academy: Chilling Water Problem
An example of how ice can cause energy to transfer and reduce the temperature of water. [11:23]
Khan Academy
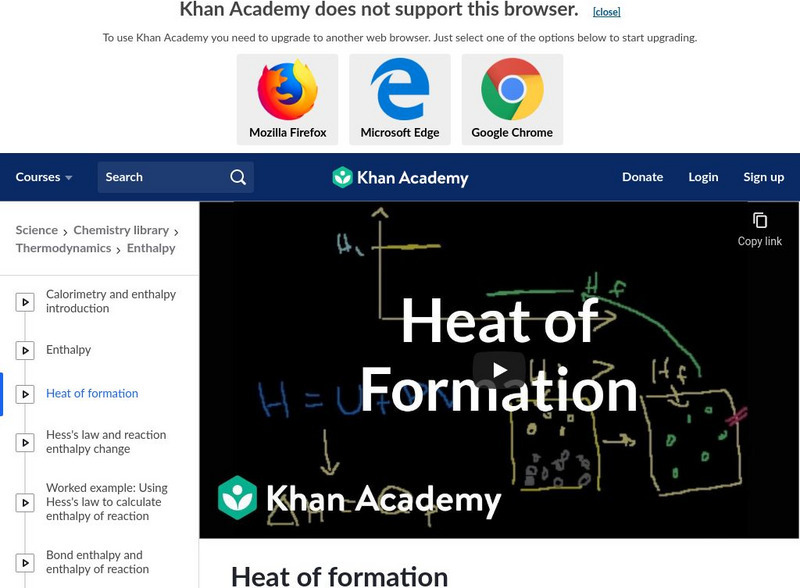
Khan Academy: Heat of Formation
An explanation to show how heats of formation are calculated. [12:35]
Khan Academy
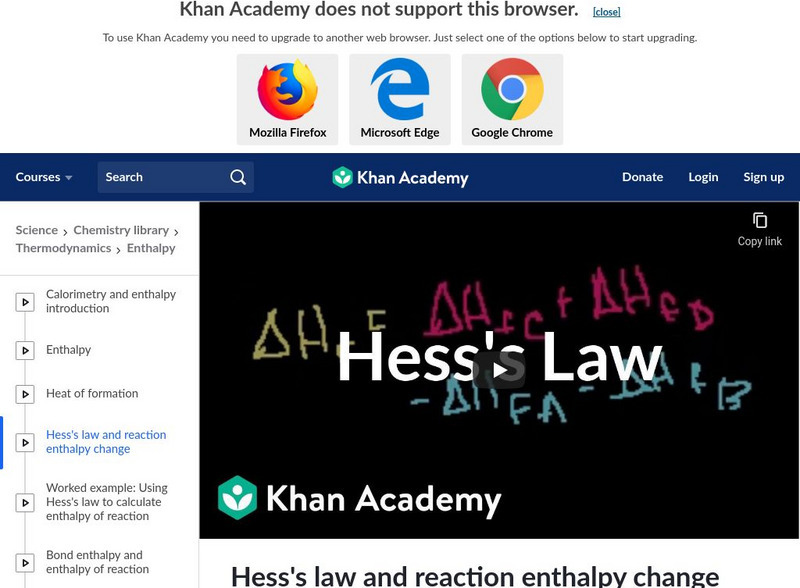
Khan Academy: Hess's Law and Reaction Enthalpy Change
Using Hess's Law and standard heats of formation to determine the enthalpy change for reactions. [15:40]
Khan Academy
Khan Academy: Hess's Law Example
An example using Hess's Law to calculate enthalpy change. [12:08]
Khan Academy
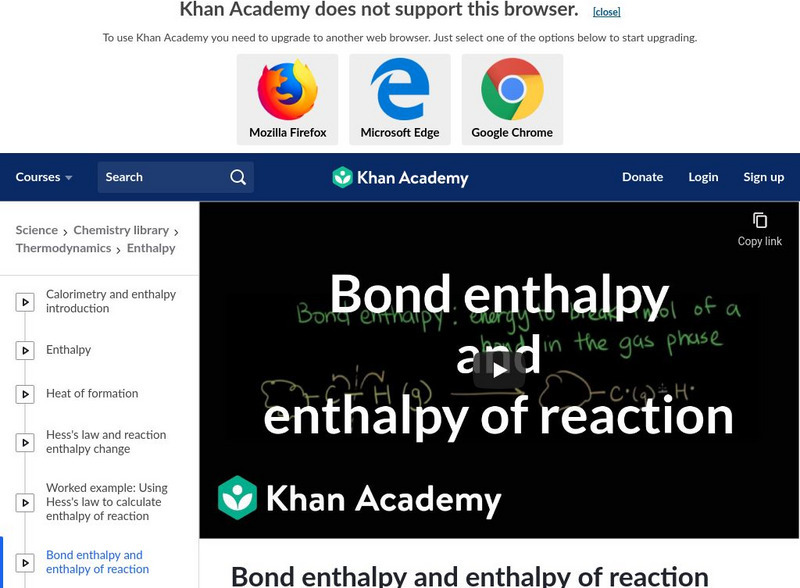
Khan Academy: Bond Enthalpy and Enthalpy of Reaction
An explanation of using bond enthalpy to calculate enthalpy of reaction. [11:47]
Khan Academy
Khan Academy: 2015 Ap Chemistry Free Response 7
Calculating the necessary energy to recycle aluminum from aluminum oxide which was a question on the 2015 AP Chemistry exam. [6:16]
Khan Academy
Khan Academy: Proof: Volume Ratios in a Carnot Cycle
An explanation to prove the volume ratios in a Carnot cycle. [17:08]
Khan Academy
Khan Academy: Proof: S (Or Entropy) Is a Valid State Variable
An explanation to prove that entropy, labeled as S, is a valid state variable. [15:38]
Khan Academy
Khan Academy: More on Entropy
An explanation showing the differences between microstates and macrostates emphasizing that entropy is defined for a system and not different microstates. [8:56]
Khan Academy
Khan Academy: Maxwell's Demon
Explanation of a concept that seems to defy the Second Law of Thermodynamics. [13:27]
Khan Academy
Khan Academy: Gibbs Free Energy Example
Calculating change in Gibb's free energy to identify whether or not a reaction is spontaneous. [9:56]
Khan Academy
Khan Academy: More Rigorous Gibbs Free Energy / Spontaneity Relationship
Extended explanation of the effect of a negative charge on Gibbs Free Energy. [13:56]
Khan Academy
Khan Academy: A Look Ar a Seductive but Wrong Gibbs/spontaneity Proof
An explanation why many textbooks are incorrect in their details about the "proof" of the relation between Gibbs free energy state and spontaneity. [6:41]
Khan Academy
Khan Academy: Changes in Free Energy and the Reaction Quotient
An explanation of Gibbs Free Energy and the reaction quotient under conditions that are not standard. [15:47]
Khan Academy
Khan Academy: Thermodynamic Entropy Definition Clarification
Using a reversible system to clarify the definition of entropy in thermodynamics. [15:38]
Khan Academy
Khan Academy: Entropy Intuition
An introduction to the Second Law of Thermodynamics focusing on terms of heat and microstates. [19:15]
PBS
Pbs Learning Media: Energy Transfer in a Trebuchet
On NOVA, a team of carpenters, timber framers, engineers, and historians recreate a medieval throwing machine called a trebuchet. This adapted video segment explores how understanding energy transfer informs their design. [4:17]
Other
Conservation of Energy in Waves
High school teacher Kate Nichols explains that wave energy is equal to the amplitude of a wave. Looks at different scenarios where we may notice that wave energy is being conserved or not. The conversion of wave energy into another type...
OpenSciEd
Open Sci Ed: 6.2 Thermal Energy: Teacher Playlist
This teacher playlist demonstrates the Thermal Energy unit investigations where the students find that there are two ways to transfer energy into a drink: (1) the absorption of light and (2) thermal energy from the warmer air around the...
OpenSciEd
Open Sci Ed: 6.2 Thermal Energy: Student Playlist
This student playlist demonstrates the Thermal Energy unit investigations where the students find that there are two ways to transfer energy into a drink: (1) the absorption of light and (2) thermal energy from the warmer air around the...
Bozeman Science
Bozeman Science: Heating
In the following video Paul Andersen explains how heating is the transfer of energy (heat) from a warmer object to a cooler object. Heat can be transferred through conduction, convection and radiation. At the microscopic level conduction...
Bozeman Science
Bozeman Science: Heat Exchange
In the following video Paul Andersen explains how energy can be transferred from warmer objects to colder objects through heat. Temperature is a measure of the average kinetic energy of the particles in a substance. When two objects are...
Bozeman Science
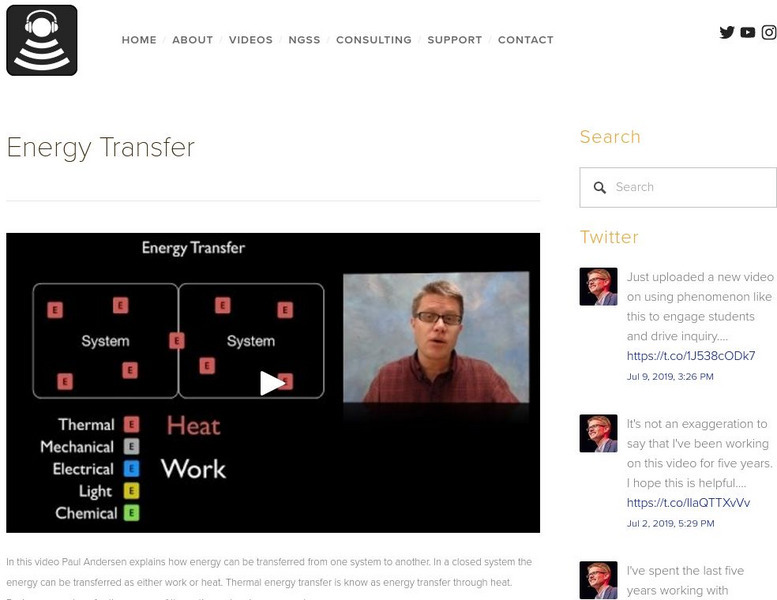
Bozeman Science: Energy Transfer
In the following video Paul Andersen explains how energy can be transferred from one system to another. In a closed system the energy can be transferred as either work or heat. Thermal energy transfer is know as energy transfer through...
Bozeman Science
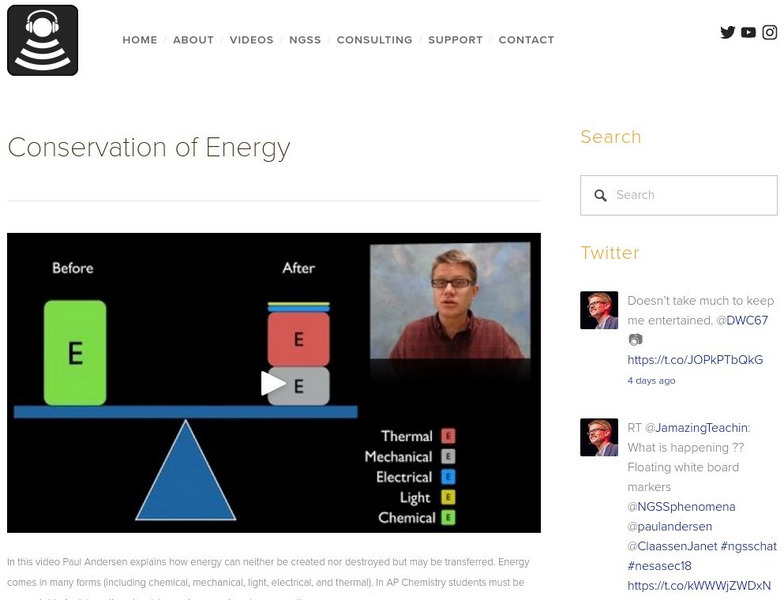
Bozeman Science: Conservation of Energy
In the following video, Paul Andersen explains how energy can neither be created nor destroyed but may be transferred. Energy comes in many forms. [4:09]