Khan Academy
Khan Academy: Chemistry: Gibbs Free Energy Example
An explanation of how to determine whether or not a reaction is spontaneous based on the change in Gibbs free energy. [9:57]
Bozeman Science
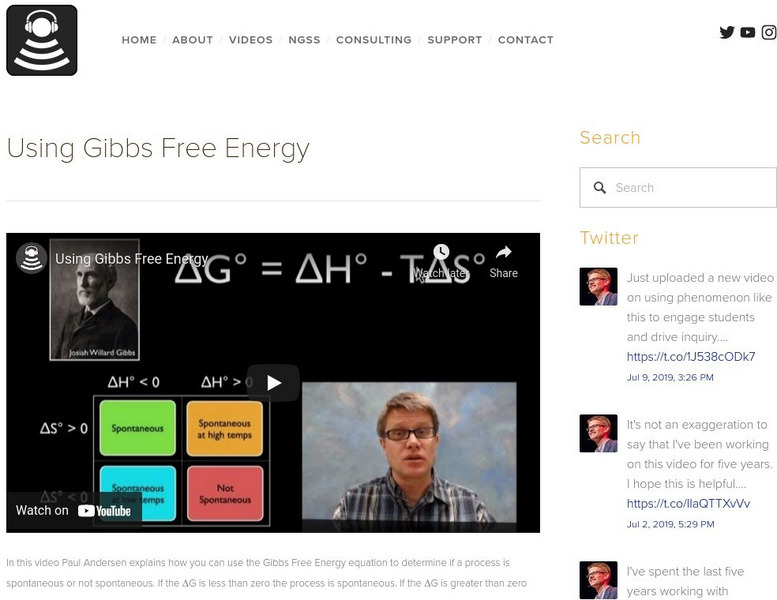
Bozeman Science: Using Gibbs Free Energy
In this video, Paul Andersen explains how you can use the Gibbs Free Energy equation to determine if a process is spontaneous or not spontaneous. Check out the concept map and slideshow to enhance study. [7:57]
Khan Academy
Khan Academy: 2015 Ap Chemistry Free Response 2 C
Do you need to review Gibbs free energy and equilibrium constant of dehydration reaction for the AP Chemistry exam? This video works a free response question from the AP Chemistry exam. [12:22]
Khan Academy
Khan Academy: Nernst Equation
Deriving a few different forms of the Nernst equation, the relationship between Gibbs free energy and reaction quotient Q. [6:32]
Khan Academy
Khan Academy: Endergonic, Exergonic, Exothermic, and Endothermic
Learn how endergonic, exergonic, exothermic, and endothermic reactions play a role in biological reactions. [11:52]
Khan Academy
Khan Academy: Changes in Free Energy and the Reaction Quotient
This video lesson from Khan Academy is intended for students who are taking the AP Chemistry course. The relationship between Gibbs free energy, standard Gibbs free energy, and the reaction quotient Q. Predicting the direction of the...
Khan Academy
Khan Academy: A Look at a Seductive but Wrong Gibbs Spontaneity Proof
This video looks at why the 'proof' of the relation between changes in Gibbs Free Energy and Spontaneity is wrong in many textbooks. [6:40]
Khan Academy
Khan Academy: Test Prep: Mcat: Biomolecules: Overview of Metabolism: Atp Hydrolysis: Gibbs Free Energy
How does ATP provide energy for biosynthesis reactions? Use an understanding of Gibbs Free Energy to understand how ATP is coupled to energy-requiring processes. [7:41]
Khan Academy
Khan Academy: Test Prep: Mcat: Chemical Processes: Bioenergenetics: An Analogy for Gibbs Free Energy
Offers an explanation of Gibbs free energy using an analogy. [9:19]
Bozeman Science
Bozeman Science: Gibbs Free Energy
Paul Andersen attempts to explain Gibbs Free Energy. He begins by using three spontaneous reactions to explain how a change in enthalpy, entropy and temperature can affect the free energy of a system. He then applies this concept to...