Mazz Media
Heat of Fusion
In this video viewers will learn that heat of fusion is equal to the amount of heat that must be added or given off to melt or freeze one gram or kilogram of a substance and it’s expressed J/g or J/kg. Students will learn what units are...
Professor Dave Explains
Phase Changes, Heats of Fusion and Vaporization, and Phase Diagrams
What the heck is dry ice and why is it so spooky? Learn this and more when we investigate phase changes and phase diagrams!
Khan Academy
Chilling Water Problem, States of Matter and Intermolecular Forces, Chemistry
Sal continues this short series on specific heat and vaporization. The situation he poses uses 500 grams of liquid water at 60 degrees Celsius to get the water to zero degrees Celsius. The ice he adds to the water comes out at -10...
SciShow
Why Are the Inner and Outer Planets Different?
How did the planets form? An interesting video from the SciShow Space series identifies the differences between the inner and outer planets and how the history of the solar system's sun put everything in its place. Viewers also learn...
Teacher's Pet
Heat in Changes of State
Melting ice seems pretty easy, right? But what's actually happening is much more complex! Introduce the class to enthalpy calculations using a video tutorial. The narrator explains and performs the calculations that show the energy at...
Khan Academy
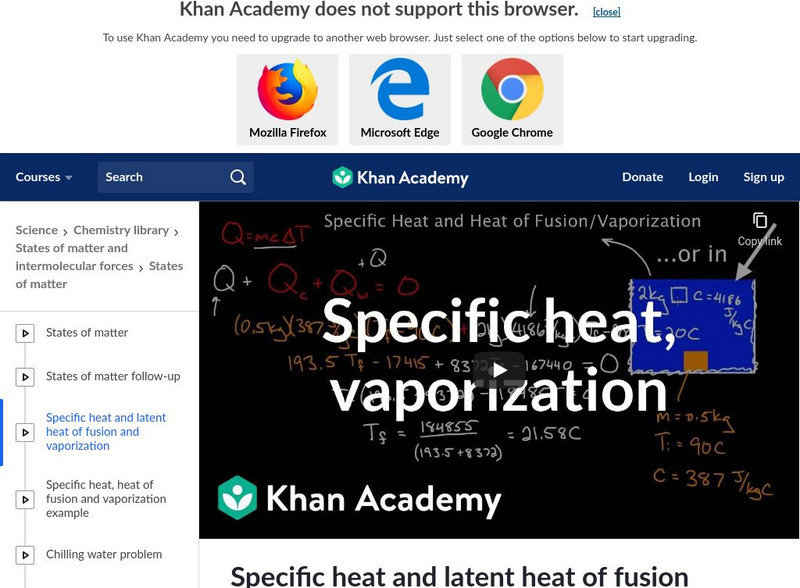
Khan Academy: Specific Heat and Latent Heat of Fusion and Vaporization
Defining specific heat, heat of fusion, and heat of vaporization, learn how to calculate the amount of heat to change the temperature of water and the energy required to change for a phase change. [14:57]
Khan Academy
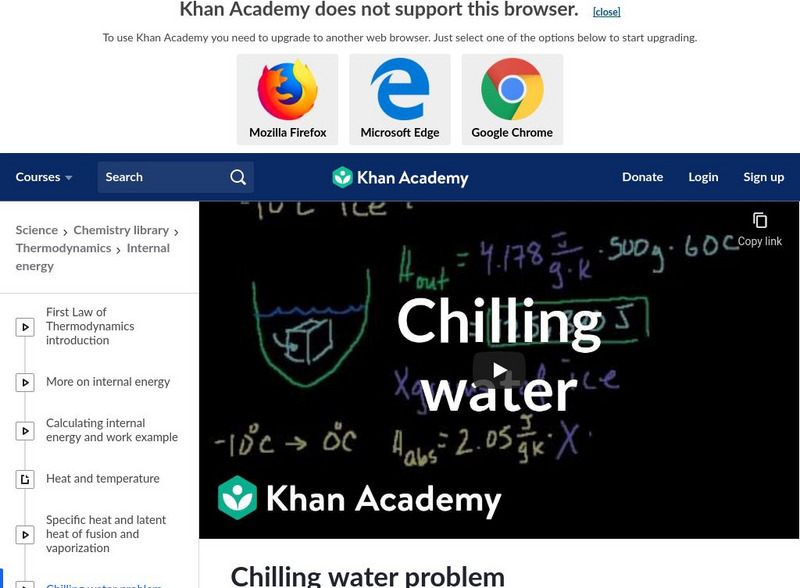
Khan Academy: Chilling Water Problem
An example of how ice can cause energy to transfer and reduce the temperature of water. [11:23]
University of Florida
Chemistry 2041 Lecture Notes: Phases and Equilibrium
An extensive set of notes and graphics (excellent graphics) pertaining to the phases of matter, phase changes and the energy changes associated with changes in phase. Characteristic properties of solids, liquids and gases are discussed....
Sophia Learning
Sophia: Enthalpy Calculation
This lesson will provide an example of calculating the total amount of energy required for water to change from a solid at a temperature below zero celsius to a gas at a temperature above 100 degrees. A labeled phase change diagram will...
Khan Academy
Khan Academy: Chemistry: Chilling Water Problem
Figure out how many grams of ice is needed to lower the temperature of water by sixty degrees in this video lecture. The lecture explains how the ice absorbs heat from the water to lower the temperature. Using the specific heat of water...
Khan Academy
Khan Academy: Chemistry: Specific Heat, Heat of Fusion and Evaporation
The concept of specific heat capacity and the heat of fusion are both reviewed in this video. [14:49]