Hi, what do you want to do?
Curated Video
Sp Hybridization in Alkynes: Exploring Triple Bonds
In alkynes, such as acetylene (C₂H₂), carbon atoms undergo sp hybridization. This involves the mixing of one 2s orbital and one 2p orbital, forming two sp hybrid orbitals. These orbitals align in a linear arrangement with a bond angle of...
Curated Video
Sp² Hybridization in Ethene: The Secret of Double Bonds
Hybridization Process: Carbon atoms undergo sp² hybridization, mixing one 2s and two 2p orbitals to form three sp² hybrid orbitals arranged in a trigonal planar geometry (120° bond angles). Bond Formation: Two sp² orbitals form sigma (σ)...
Curated Video
VSEPR Theory - lone electron pair influence
Bond Angle Distortion: Lone pairs reduce bond angles between bonding pairs. For example, in a molecule like NH₃ (ammonia), the ideal tetrahedral angle of 109.5° is compressed to around 107° due to the presence of a lone pair on nitrogen....
Curated Video
Shaping Molecules: Geometry of AB₄, AB₅, and AB₆ Explained
The geometry of molecules with the general formulas AB₄, AB₅, and AB₆ is determined by the Valence Shell Electron Pair Repulsion (VSEPR) theory, which helps predict molecular shapes based on the repulsion between electron pairs around...
Curated Video
VSEPR Theory Postulates: The Rules Behind Molecular Geometry
The Valence Shell Electron Pair Repulsion (VSEPR) theory predicts molecular shapes by focusing on the repulsion between electron pairs surrounding a central atom. The main postulates include: Electron Pair Repulsion: Electron pairs...
Curated Video
VSEPR Theory: Predicting Molecular Shapes with Ease
VSEPR postulates state that the geometry of a molecule depends on the number and arrangement of bonding and lone electron pairs around the central atom
Curated Video
Geometry in BeCl₂ & BF₃: A VSEPR Theory Perspective
BeCl₂ and BF₃ exhibit linear and trigonal planar geometries, respectively, as predicted by the VSEPR theory, minimizing electron pair repulsion
Curated Video
VSEPR Theory: Learn Molecular Geometry Fast - Chemistry Study Guide
Struggling with VSEPR theory and molecular geometry? This video simplifies the concepts you need to master these essential chemistry topics. Learn how to draw Lewis structures with the correct geometry, understand bond angles, and...
Professor Dave Explains
Limitations of VSEPR Theory
We've learned about VSEPR theory, and we know how to use it to predict molecular geometry for a variety of organic molecules. But in fact, there are situations where predictions made with VSEPR theory do not line up with experimental...
Professor Dave Explains
Practice Problem: VSEPR Theory and Molecular Geometry
What's with all these shapes? Let's practice assigning hybridization, electron-domain geometry, and molecular geometry. Octahedral! Tetrahedral! Linear! Pyramids and see-saws and what not! Chemistry is fun, isn't it?
Professor Dave Explains
IIT/JEE Chemistry Practice #20: Hybridization
Practice REAL problems from actual past IIT/JEE exams with Professor Dave!
Professor Dave Explains
IIT/JEE Chemistry Practice #17: Molecular Geometry
Practice REAL problems from actual past IIT/JEE exams with Professor Dave!
Professor Dave Explains
Visualizing Molecular Geometry With 3D Software
We've already learned about VSEPR theory, and how to use it to predict the shapes of various molecules. But we didn't cover all of the molecular geometries for certain hybridizations, and we didn't talk about bond angles, so let's do...
Professor Dave Explains
Crystal Field Theory
We are used to using a theory like VSEPR theory to predict molecular geometry, but unfortunately with coordination compounds, things are not so simple, because of those pesky d orbitals on the central metal atom. Crystal field theory is...
Professor Dave Explains
VSEPR Theory and Molecular Geometry
Did you know that geometry was invented by molecules? It's true! Until the first stars went supernova and littered all the elements across the cosmos, everything was simply spheres, from protons to stars. But then, under cooler planetary...
JFR Science
VSEPR Theory: Determining the 3D Shape of Molecules
Ready to take molecules out of the two dimensional world and into 3-D? Chemistry scholars explore molecular geometry through a well-written video from the JFR Science series. Topics include the effects of bonding and non-bonding...
Berkeley University of California
Oxidation Bond Angle (NB)
What is the show Caesium and Iodine love watching together? CsI. The first video explains how to determine the bond angle of an oxidation. The second focuses on how to determine which are linear ions by using molecular geometry.
Khan Academy
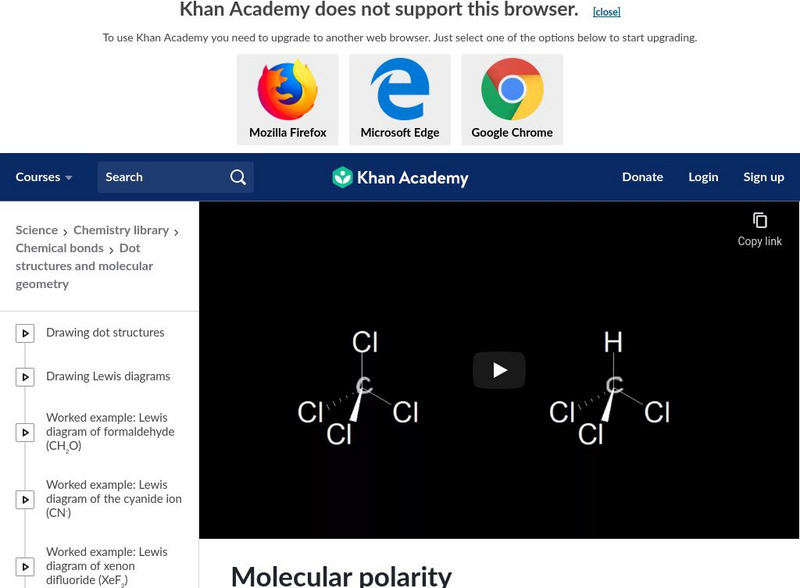
Khan Academy: Dipole Moment
Learn how to predict the molecular dipole moment based on the molecular geometry. [9:21]
Khan Academy
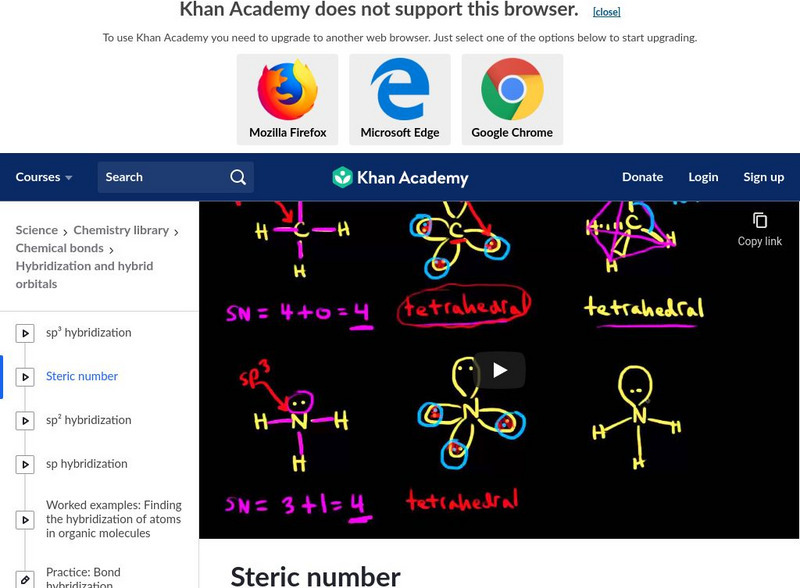
Khan Academy: Steric Number and Sp3 Hybridization
Find out how to use steric number to calculate the number of hybrid orbitals. [9:47]
Khan Academy
Khan Academy: Organic Hybridization Practice
Examples of identifying hybridization and geometry for atoms in organic molecules. [10:20]
Sophia Learning
Sophia: Vsepr: Basic Geometry
A narrated lesson introducing the five basic shapes of simple molecules. [2:47]
Sophia Learning
Sophia: Vsepr: Bent Molecules
A video lesson introducing the concept of bent shaped molecules including bond angles. [4:26]
Khan Academy
Khan Academy: Triple Bonds Cause Linear Configurations
Understand the linear geometry of a triple bond. [1:11]
Sophia Learning
Sophia: Vsepr: Bent Molecules: Lesson 2
This lesson will explore bent shaped molecules including bond angles. It is 2 of 2 in the series titled "VSEPR: Bent Molecules."