Simon Fraser University
Chem1 Virtual Textbook: Sizes of Atoms and Ions
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site seeks to answer the question, What do we mean by the "size" of an atom? Terms such as metallic radius, covalent radius,...
Wikimedia
Wikipedia: Democritus
This encyclopedia entry surveys the life and thought of the 5th century BCE Greek philosopher Democritus, who proposed the existence of the atom.
Museum of Science
The Atoms Family: Spectroscope of an Atom
"The Phantom has provided you with a simulated spectroscopy of an Atom! Amuse the Phantom by observing the spectroscope and you'll learn more about the Atom." Observe and review the structure of the atom.
Wikimedia
Wikipedia: Absolute Zero
Wikipedia offers several paragraphs of detailed information on absolute zero, the lowest temperature that can be obtained in any macroscopic system.
Wikimedia
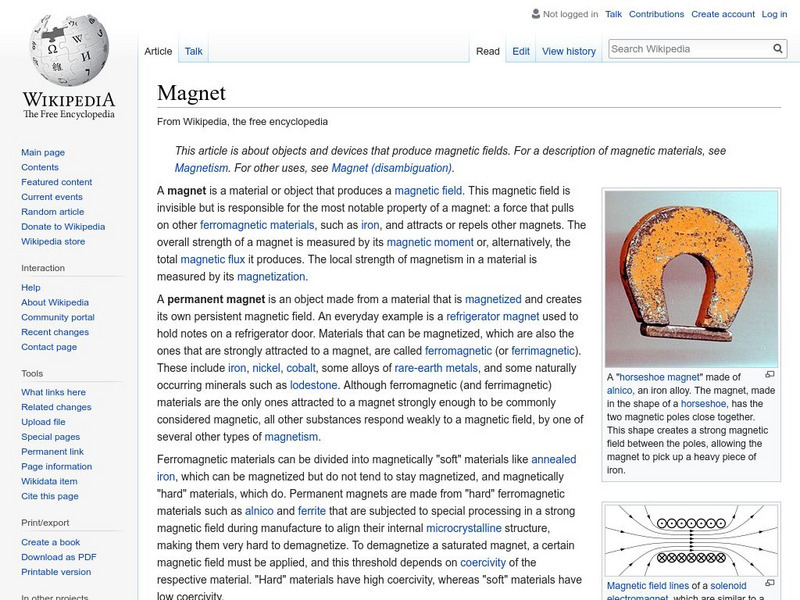
Wikipedia: Magnet
Wikipedia.com provides an excellent introductory site on magnets. Including basic information describing different types of magnets and their characteristics.
Estrella Mountain Community College
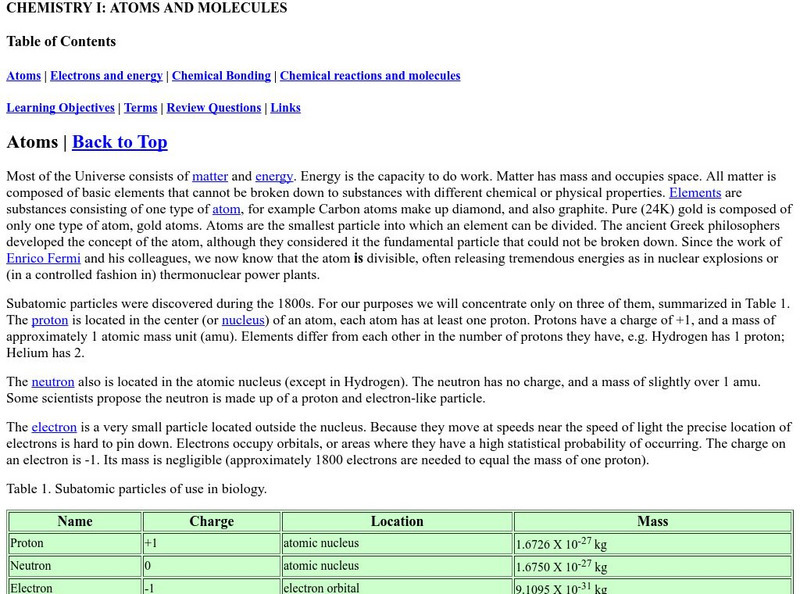
Online Biology Book: Chemistry I: Atoms and Molecules
In this online biology textbook, learn about atoms and molecules as they relate to life. Find out about topics such as electrons and energy, chemical bonding, and chemical reactions.
Upper Canada District School Board
Tom Stretton's Advanced Placement Chemistry: Atomic Structure and Periodicity
This chemistry e-textbook provides students with AP-level reading and practice material on atomic structure and periodicity.
TED Talks
Ted: Ted Ed: If Matter Falls Down, Does Antimatter Fall Up?
Chloe Malbrunot investigates matter and anti-matter by placing two atoms- one made of matter, and the other antimatter- in the cockpit of a plane, ready to jump. What do you think will happen? [2:54]
Open Curriculum
Open Curriculum: The Atom
Learn about atoms and their properties of motion with this illustrated article.
Thomas Jefferson National Accelerator Facility
Jefferson Lab: Atoms, Elements and Molecules: Questions and Answers
Read common questions and answers about atoms, elements and molecules.
American Museum of Natural History
American Museum of Natural History: O Logy: Stuff to Do: Atomic Mobile
Illustrated instructions for how to make a model of an atom (an atom mobile).
Thomas Jefferson National Accelerator Facility
Jefferson Lab: Element Matching Game
Use this resource to practice memorizing the names for the chemical symbols. This interactive resource has a function that will check your answers.
Thomas Jefferson National Accelerator Facility
Jefferson Lab: Reading Passages: Charges and Electricity
Read and fill in the blanks of this passage explaining charges and electricity. Each blank has a dropdown menu with choices. When you finish, click CHECK MY ANSWERS. If you pick a wrong answer, the right answer will be displayed along...
Thomas Jefferson National Accelerator Facility
Jefferson Lab: Reading Passages: Looking for Quarks Inside the Atom
Read and fill in the blanks of this passage explaining quarks inside the atom. Each blank has a dropdown menu with choices. When you finish, click CHECK MY ANSWERS. If you pick a wrong answer, the right answer will be displayed along...
PBS
Nova: The Atom Builder
A brief explanation is provided for designing a stable atom. You can also refer to a labeled model of a carbon atom. This resource also has a link to an atom building activity.
PBS
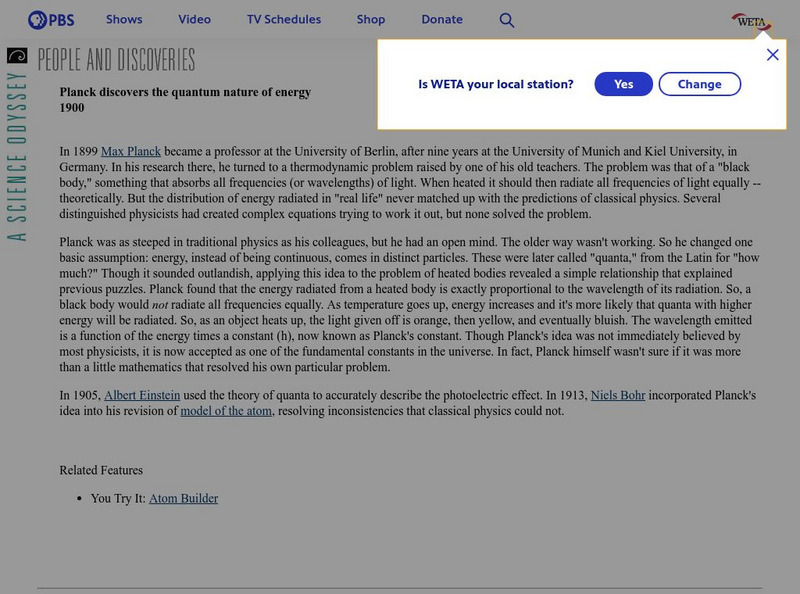
Pbs: Planck Discovers the Quantum Nature of Energy
PBS offers a short summary of the discovery of the quantum nature of the atom by Max Planck. Easy to follow.
Lawrence Berkeley National Laboratory
Berkeley Lab: Particle Adventure: The Standard Model
An introduction to the Standard Model, a theory which attempts to explain atomic structure using leptons, quarks, and force carrier particles.
Other
Classic Chemistry: Jean Perrin
An excerpt of Perrin's paper "Brownian Motion and Molecular Reality". Includes references.
Other
Le Moyne University: John Dalton
This site from the Le Moyne University provides excerpts from Dalton's "A New System of Chemical Philosophy" published in 1808. Includes Dalton's table of atomic weights and and scanned atomic symbols.
Other
University of Kansas: Quarked!: Matter Mechanic
Build elements and molecules using neutrons, protons, and electrons. Choices include helium, carbon, oxygen, aluminum, water, and salt.
Lawrence Berkeley National Laboratory
Berkeley Lab: La Aventura De Las Particulas
Learn the fundamentals of particles and forces with this site. Explore the paths that explain matter in the universe.
Other
Siyavula Education: Everything Maths & Science: Models of the Atom
Discusses models of the atom developed by John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick. Includes clear illustrations and a short comprehension exercise at the end.
Texas Education Agency
Texas Gateway: Atoms, Elements and the Periodic Table: The Atomic Model [Pdf]
A slideshow looking at the contributions of scientists over time to our understanding of atomic theory. Looks at models of the atom developed by Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick, as...
Other
Cronodon: Atoms Models of the Atom
Presents models of the atom developed by Thomson, Rutherford, Bohr, and Schrodinger. Includes successes and failures of each, illustrations, and detailed descriptions. Also discusses Dirac's model of the atom and introduces quantum...