Khan Academy
Khan Academy: Collision Theory
An introduction to collision theory and activation energy is presented. [8:42]
Khan Academy
Khan Academy: Arrhenius Equation
Definition of rate constant k, the pre-exponential factor A, and activation energy are introduced. Understand how the exponential part of the Arrhenius equation depends on activation energy and temperature. [9:21]
Khan Academy
Khan Academy: Forms of the Arrhenius Equation
Know how to write different forms of the Arrhenius equation. Use the Arrhenius equation to look at how changing temperature and activation energy affects collisions. [6:36]
Khan Academy
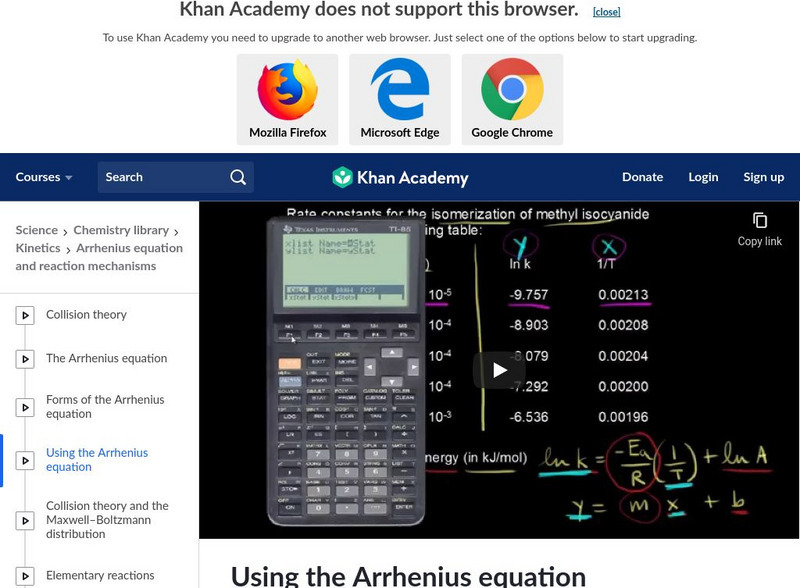
Khan Academy: Using the Arrhenius Equation
Know how to use the Arrhenius equation to calculate the activation energy. [11:01]
Khan Academy
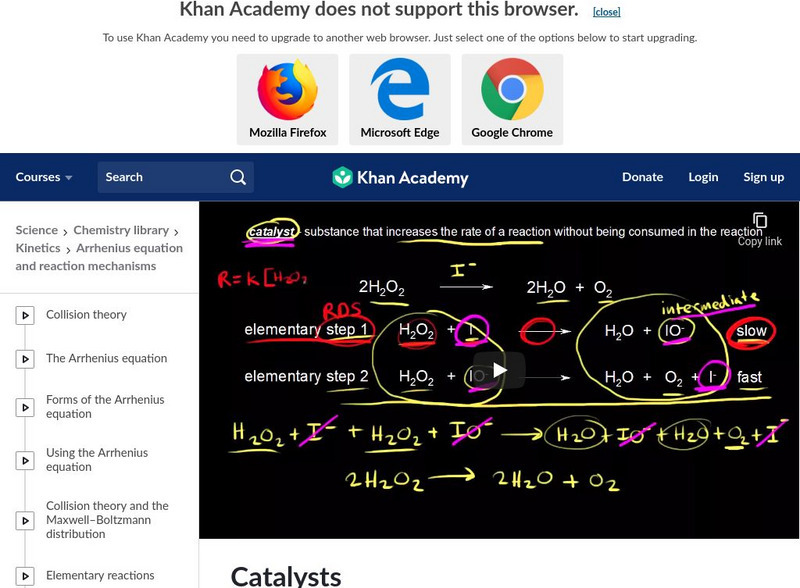
Khan Academy: Catalysts
Sal will illustrate how a catalyst speeds up a reaction by lowering the activation energy. [7:24]
Khan Academy
Khan Academy: Energy & Enzymes: Introduction to Enzymes
Learn about the function of enzymes in biological systems in this video. Video overviews active sites, substrates, induced fit, and activation energy. [8:12]
Sophia Learning
Sophia: Definition of a Catalyst
This lesson will define the term catalyst and explain how a catalyst affects the activation energy of a chemical reaction. Examples provided.
Sophia Learning
Sophia: Energy Diagram of a Chemical Reaction
This lesson will introduce the energy diagram of a chemical reaction, and label the parts of the diagram.
Bozeman Science
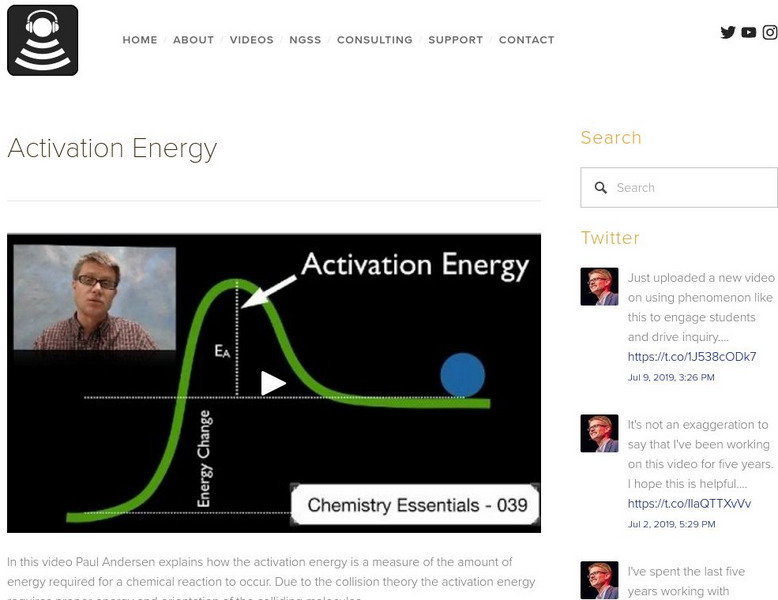
Bozeman Science: Activation Energy
In this video Paul Andersen explains how the activation energy is a measure of the amount of energy required for a chemical reaction to occur. Due to the collision theory the activation energy requires proper energy and orientation of...
Sophia Learning
Sophia: Chemical Reactions: Summary: Lesson 2
This lesson will discuss how chemical reactions occur and that not all of them will go through to completion. It is 2 of 2 in the series titled "Chemical Reactions: Summary."
Crash Course
Crash Course Chemistry #32: Kinetics Chemistry's Demolition Derby
In this episode, Hank talks about collisions between molecules and atoms, activation energy, writing rate laws, equilibrium expressions, reactions mechanics, and rate-determining steps. [9:57]
Khan Academy
Khan Academy: Reaction Rates: Introduction to Kinetics
A video lecture that focuses on kinetics, the study of how reactions progress and the rate of reactions. Kinetics, activation energy, activated complex and catalysts are discussed. [15:27]
Khan Academy
Khan Academy: Energy and Transport: Enzymes
Enzymes as catalysts for reactions in biological systems; discussion of substrates, active sites, induced fit, and activation energy. [8:12]
Khan Academy
Khan Academy: Arrhenius Equation
The Arrhenius equation is k = Ae^(-Ea/RT), where A is the frequency or pre-exponential factor and e^(-Ea/RT) is the fraction of collisions that have enough energy to react (i.e., have energy greater than or equal to the activation energy...
Next Vista for Learning
Next Vista for Learning: Enzymes
A video describing how enzymes function as a reaction catalyst. The video shows how enzymes work through animated examples. [1:15]
Sophia Learning
Sophia: Activation Energy: Lesson 5
This lesson will define activation energy and explain how to determine the amount of activation energy from an energy diagram. It is 5 of 5 in the series titled "Activation Energy."
Sophia Learning
Sophia: Catalyst: Lesson 3
This lesson will describe that a catalyst is something which can speed up a reaction by lowering activation energy. It is 3 of 4 in the series titled "Catalyst."