Catalyst University
Combining Cations & Anions to Name Ionic Compounds
Here, I show you how to write the formula for an ionic compound given the cation and anion. [5 examples]
Catalyst University
Superoxides and Superoxide Dismutase: Physiology, Biochemistry, and Inorganic Mechanism
Superoxides and Superoxide Dismutase: Physiology, Biochemistry, and Inorganic Mechanism
Catalyst University
Oxymercuration/Demurcuration: Theory, Mechanism, and Examples
Oxymercuration/Demurcuration: Theory, Mechanism, and Examples
Professor Dave Explains
Practice Problem: Ionization Energy
When we learned about periodic trends, we learned about ionization energy. Just how much energy is required to remove an electron from an atom? What about a second electron, or a third? Let's compare a few different ionizations and see...
Professor Dave Explains
Practice Problem: Analyzing Acid-Base Equilibria
Acids! Bases! Conjugate acids! Conjugate bases! We definitely have to be able to label such things, and we should also know how to state which direction in an acid-base equilibrium is preferred. To do this we should know how to tell...
Professor Dave Explains
Types of Silicates Part 2: Inosilicates, Phyllosilicates, and Tectosilicates
Silicates are a particularly complex class of minerals that all contain silica tetrahedra. What are the characteristic structures of the last three sub classes of silicates? How are they different from one another? The 8 Classes of...
Professor Dave Explains
Practice Problem: Lattice Energy and Ionic Bond Strength
We know that within an ionic compound, the ions are held together by ionic bonds. What is the strength of those bonds, and what is the lattice energy possessed by the entire lattice? Can we compare two compounds and say which has the...
Professor Dave Explains
Organic Chemistry Mechanism Challenge 6
Need some organic chemistry practice? Here's a tricky mechanism to try!
Professor Dave Explains
Metallic Bonds
We've learned about ionic and covalent bonds, so we understand the interactions that will occur between a metal and a nonmetal, or between two nonmetals. But what about two metals? Metallic bonding! This ends up being sort of like ionic...
Professor Dave Explains
Nomenclature of Hydrated Salts
As long as we're naming stuff, let's name these hydrated salts! You know, like epsom salts?
Professor Dave Explains
Native Elements, Oxides, Halides, and Sulfides
Different types of rocks contain different classes of minerals, which can tell us a lot about the geological environment in which the rock formed. What are these different mineral classes? What is the common structure of each class? The...
Schooling Online
Chemistry Properties and Structure of Matter: Properties of Matter - Naming Monoatomic Ions
This lesson will introduce the rules for writing the names and chemical formulae of non-metal anions and metal cations, including metals with multiple positive oxidation states.
Definitions included: compound, cation, anion, IUPAC,...
FuseSchool
Electrolysis Of Molten Compounds
Learn the basics about Electrolysis of Molten Compounds. What is electrolysis? What are molten compounds? Find out more in this video!
FuseSchool
What Are Salts?
Learn the basics about what salts are, as part of the overall topic of acids and bases.
Professor Dave Explains
Aromaticity and Huckel's Rule
What is it for a molecule to be aromatic? Where was this term derived and what properties does it bestow upon a molecule?
FuseSchool
What Are The Reactions Of Halogens
Learn the basics about the reactions of halogens, when learning about the periodic table as a part of properties of matter. Group 7 of the periodic table is the halogens. The reaction between diatomic chlorine gas and cold sodium...
Mazz Media
Naming Ionic Compounds
This video begins with an example of a simple ionic compound, salt, showing a model and then its chemical name. The video continues with an example of a divalent metal and discusses the oxidation number of the elements in these compounds...
Professor Dave Explains
Naming Ionic Compounds
We have to know how to name ionic compounds. Not any name we want like Jeff or Larry, there's rules for how to name them. And look at all these adorable polyatomic ions!
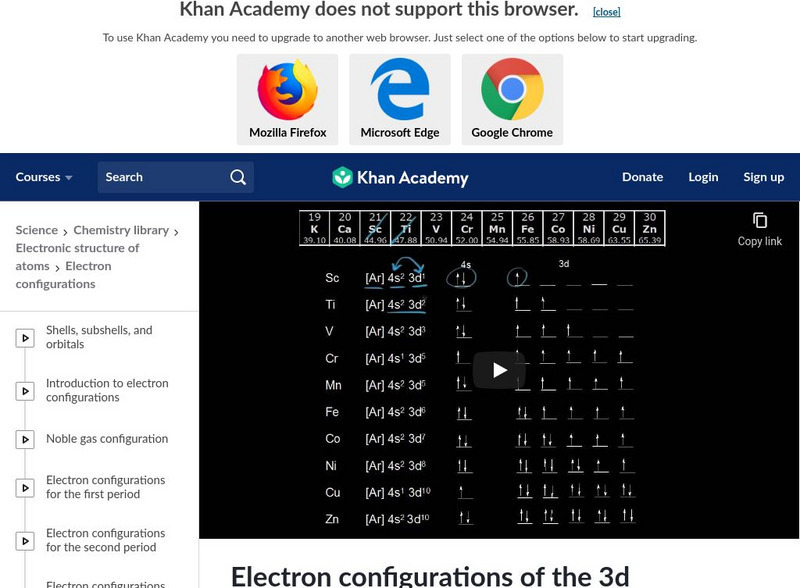
Khan Academy
Khan Academy: Electron Configurations in the 3d Orbitals
A review of the electron configurations and orbital notations with a focus on K, Ca, and Sc. [12:33]