Hi, what do you want to do?
Curated OER
Typical Conceptual Questions for Physics I - Heat
This worksheet would make a nifty quiz on the laws of thermodynamics. Nine multiple choice questions assess high schoolers' understanding of energy transfer, specific heat capacity, phase change, fusion, and vaporization. It is short but...
Georgia State University
Georgia State University: Hyper Physics: Heat of Vaporization
A discussion of the vaporization process and the energy changes which accompany the process. Includes an informative graphic and a discussion of how to determine the heat of vaporization.
Georgia State University
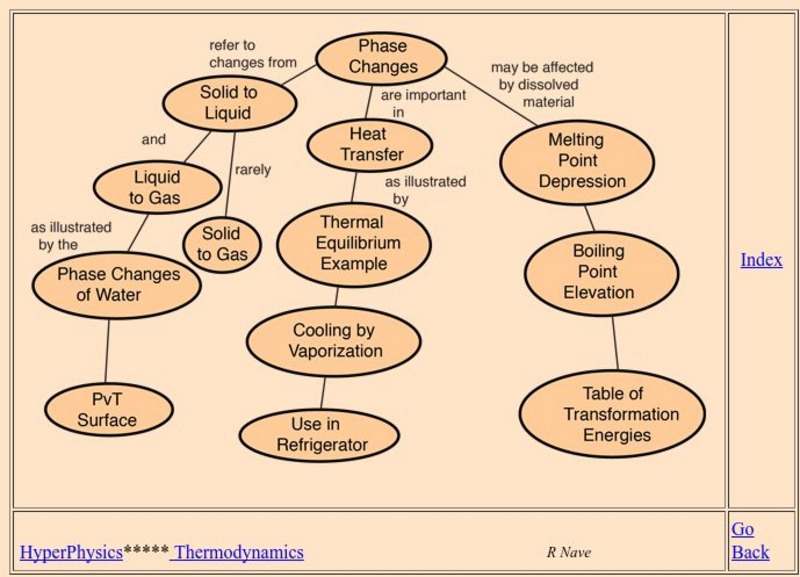
Georgia State University: Hyper Physics: Phase Change Concepts
An indexing page for the HyperPhysics site. This page includes links to a variety of pages at the site which contain information related to phase changes. Each individual page consists of informative graphics and clear explanations.
Texas Instruments
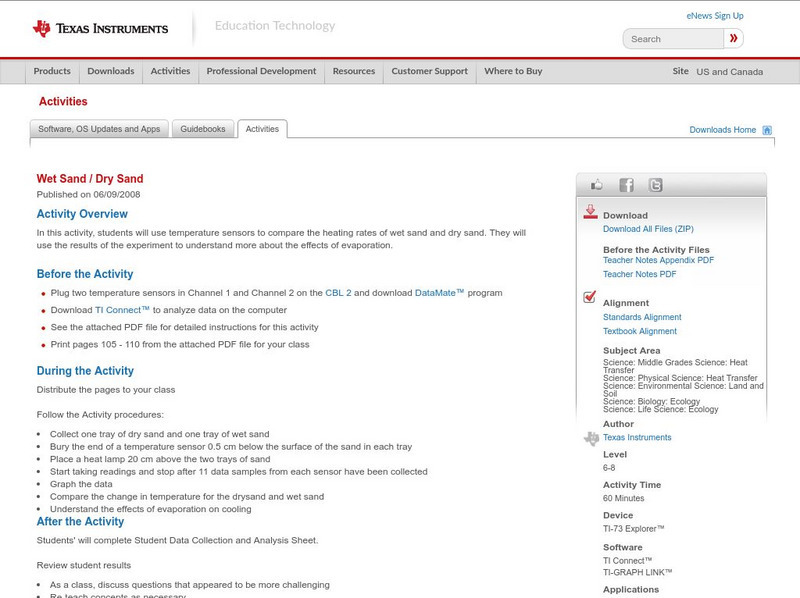
Texas Instruments: Wet Sand / Dry Sand
In this activity, Students can use temperature sensors to compare the heating rates of wet sand and dry sand. They will use the results of the experiment to understand more about the effects of evaporation.
Environmental Chemistry
Periodic Table of Elements: Gallium
A very detailed look at the element Gallium, a member of the Boron Group.
Environmental Chemistry
Periodic Table of Elements: Indium
A very detailed look at the element Indium, a member of the Boron Group.
Environmental Chemistry
Periodic Table of Elements: Thallium
A very detailed look at the element Thallium, a member of the Boron Group.
Wikimedia
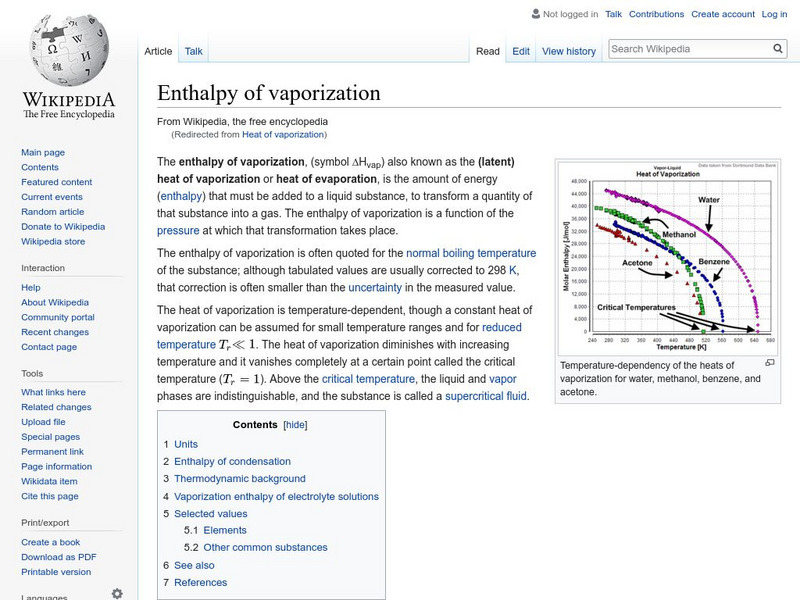
Wikipedia: Heat of Vaporization
Wikipedia offers a site on the heat of vaporization. Provides a chart of the heat of vaporization of the elements.
Encyclopedia of Earth
Encyclopedia of Earth: Physics & Chemistry: Boiling Point
A scientific explanation of what a boiling point is, the factors that can alter a liquid's boiling point, changes of state that occur at the boiling point, and how the normal boiling point relates to a liquid's vapor pressure. (Updated:...
University of Waterloo (Canada)
The University of Waterloo: The Heating Curve
The heat of vaporization along with several other thermal properties are explained. Sample problems are given. Illustrations.
Sophia Learning
Sophia: Labeling a Phase Change Diagram
This lesson will introduce a phase change diagram for water, relating energy to progress of a phase change, with labeling including specific heat values, heat of fusion value and heat of vaporization value.
Georgia State University
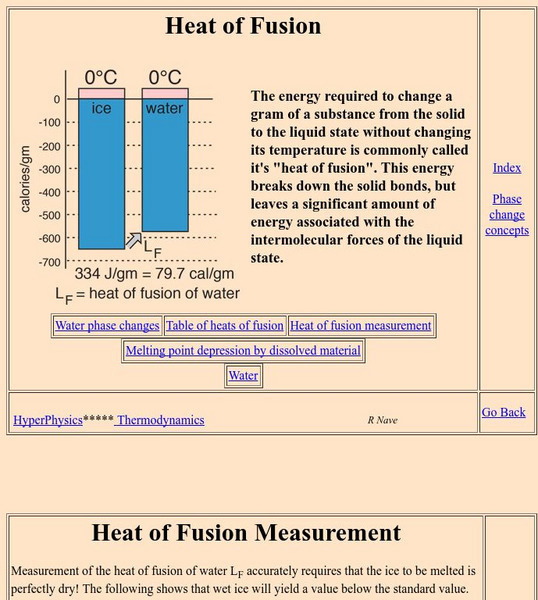
Georgia State University: Hyper Physics: Heat of Fusion
The heat of fusion is defined and described. A graphical representation of the heat of vaporization is given. A method for measuring and calculating the heat of fusion is also presented and explained.
Georgia State University
Georgia State University: Hyper Physics: Saturated Vapor Pressure
The meaning of vapor pressure is introduced. The distinction between evaporation and boiling is discussed and explained. The reason that liquids undergo vaporization is explained.
Oklahoma Mesonet
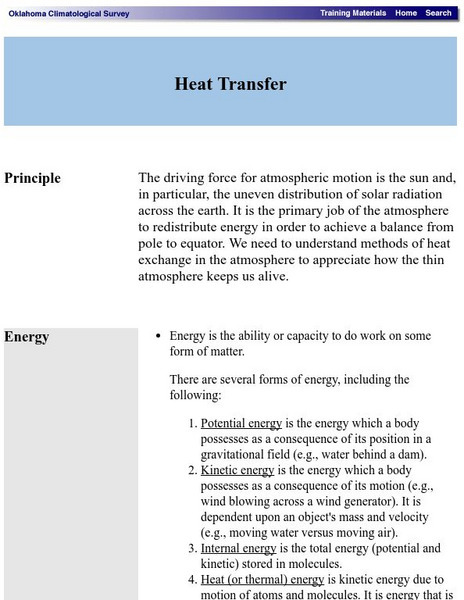
Oklahoma Climatological Survey: Heat Transfer
A discussion from the Oklahoma Climatological Survey of the thermal factors effecting the movement of air masses in the atmosphere. Numerous topics such as methods of heat transfer, latent heat, phase changes (including sublimation and...
USA Today
Usa Today: Latent Heat Supplies Weather Energy
This article provides information on what latent heat is. A good graphic is provided and links to key terms.
University of Wisconsin
University of Wisconsin Madison: Physics Demonstrations: Sourcebook: Heat
Suggestions for physics classroom demonstrations pertaining to heat and thermal energy. [Author's Note: The demonstrations and other descriptions of procedures and use of equipment in this book have been compiled from sources believed to...
Other
Homewood City Schools: Classification of Matter
This Homewood City Schools site has an outline form and contains lots of information about the classification and composition of matter. Some of the topics covered are matter and temperature, changes in state, composition of matter, and...
University of Texas at Austin
Thermo Dex:index of Selected Thermodynamic Data Handbooks
Do you need to know a specific value of a specific quantity for any given substance? If so, this is the place. Find the heat capacity, heats of vaporization and fusion, and much more from this page.
Other
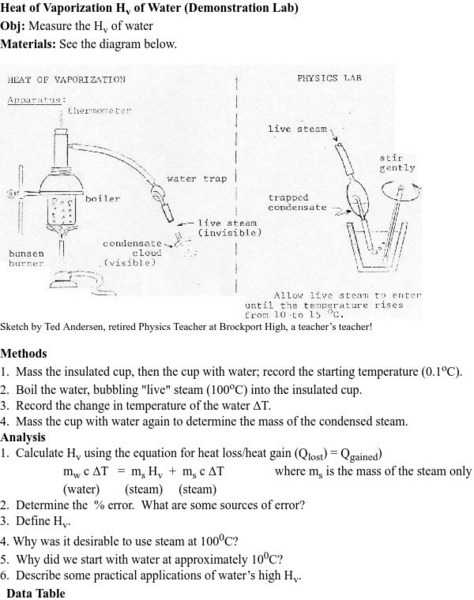
Physics Labs/heat of Vaporization (Hv) of Water
A complete set of directions, notes and suggestions for a demonstration involving the determination of the heat of vaporization of water. Suitable for a student project or lab investigation.
Purdue University
General Chemistry Topic Review/heat
Heat is defined and explained; the concept of heat is related to the kinetic motion of particles and to temperature. Heat capacity and specific heat are explained. Sensible heat is compared to latent heat; reference is made to the heat...
Wikimedia
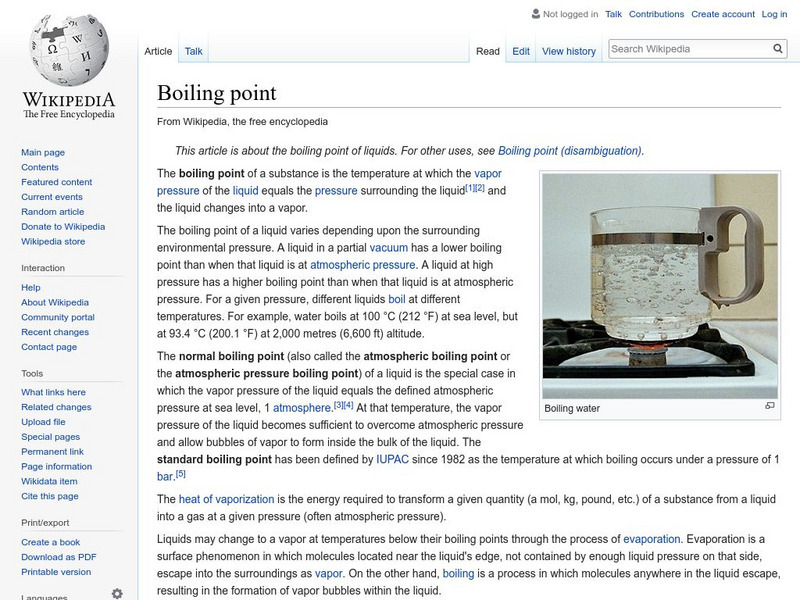
Wikipedia: Boiling Point
An encyclopedia article on boiling point explains what it is, what is needed for a substance to reach a boiling point, and what latent heat of vaporization is.
University of Sydney (Australia)
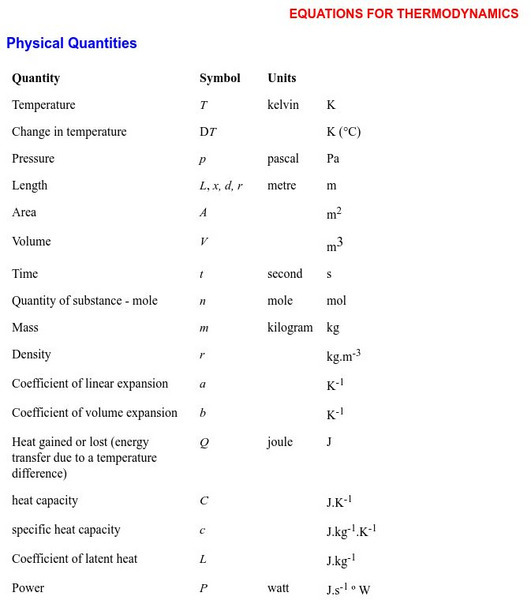
Equations for Thermodynamics
An exhaustive list of equations and formulas which are commonly used in thermal physics (including equations for triple point). Equations are organized according to category. Meaning of the symbols is clearly stated.