Hi, what do you want to do?
Curated OER
Quantitative Problems (Mass)
In this chemistry worksheet, learners identify how much aluminum and hydrochloric acid is needed to produced a certain amount of hydrogen gas. Then they identify how many grams of oxygen are used.
Curated OER
Production of Hydrogen
Students generate hydrogen gas and examine some of its properities through an experiment. In the experiment, a wood splint is lit along with a candle, then the reaction occurs within the mixture. There are questions for the student to...
University of Colorado
University of Colorado: Physics 2000: Spectral Lines
Several pages from an excellent site which describe the science of spectroscopy. The unique atomic emission (and absorption) line spectrum of elements are illustrated and explained. Includes a Java applet depicting the quantum energy...
Vision Learning
Vision Learning: Nuclear Fusion
Interactive concept simulation demonstrates the nuclear fusion of deuterium and tritium inside a tokamak reactor.
Georgia State University
Georgia State University: Hyper Physics: Hydrogen Energies and Spectrum
This site from Georgia State University gives information on the transitions of electrons between energy levels. The energy levels for electrons in the hydrogen atom are discussed. The Rydberg equation is stated and electron transitions...
University of Colorado
University of Colorado: Physics 2000: Balmer's Formula
A short description of a physics lab involving the determination of the wavelength of the four spectral lines in the hydrogen emission spectrum. Perhaps the most important part of the page is the picture of the spectrometer.
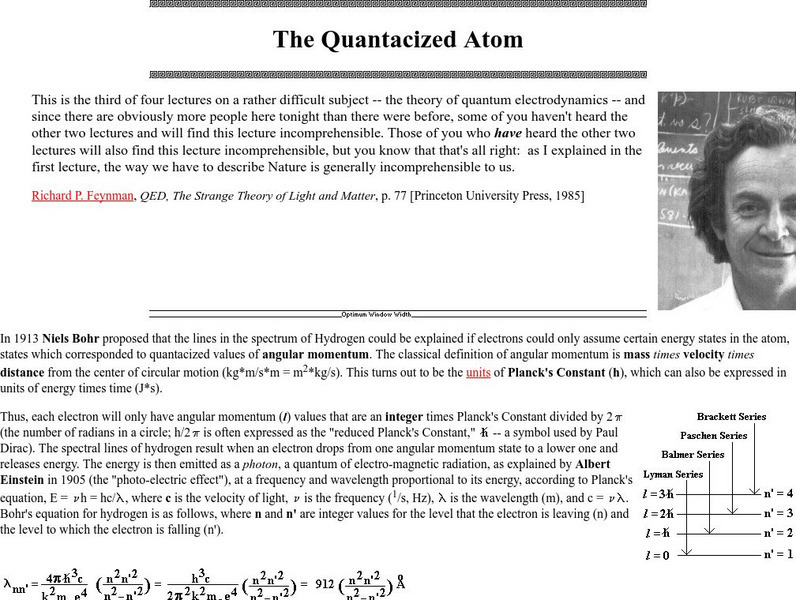
Friesian School
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
McGraw Hill
Mc Graw Hill Companies: Origins of the Quantum Theory
An indexing page for Chapter 27 (Origins of the Quantum Theory) of the companion web site for McGraw Hill's Contemporary College Physics textbook. Includes several worthy pages with chapter notes, simple definitions, online computer...
Michigan Technological University
Michigan Technological University: Henry Cavendish
An overview of scientist Henry Cavendish's accomplishments. Discusses his achievements, primarily his work with quantitative measurements.
Other
Brockport High School: Energy Levels of Hydrogen Atom
From the Brockport High School Physics Labs web pages. Includes an excellent graphic depicting the energy levels of a hydrogen atoms and portraying the electron level transitions for the Lyman, Balmer, and Paschen series. Includes both...
Hunkins Experiments
Hunkin's Experiments: How to Make Oxygen From a Battery
Hunkin's Experiments is a group of simple cartoon illustrations of scientific principles. Some would work well in the classroom, but others have little value beyond entertaining students. All of the projects are easy to do. This one...
University of St. Andrews (UK)
University of St. Andrews: Johann Jakob Balmer
Describes the life and scientific contributions of Johann Jakob Balmer. Discussion focuses on Balmer's contributions to the line spectra of hydrogen.