Hi, what do you want to do?
Khan Academy
Khan Academy: Chemistry: Reaction in Equilibrium
In this video module explore how equilibrium in a chemical reaction is defined as when the rate going forward is equal to the rate of the reverse reaction. Also learn how to calculate the equilibrium constant by knowing the concentration...
Next Vista for Learning
Next Vista for Learning: Combustion Reaction
A video showing that a combustion reaction is an exothermic reaction that releases energy in the form of fire. Video explains and demonstrates the reaction that takes place between hydrogen gas and oxygen gas. [1:55]
Science for Kids
Science Kids: Chemistry Videos: Salt and Icy Roads
Those in regions that get snow are all-too familiar with anticipation of salt trucks rolling down the roads. What purpose does the salt serve in icy conditions? [5:50]
Science for Kids
Science Kids: How to Videos: Lemon Battery
A video demonstration on how to make a lemon battery with a copper penny, galvanized nail coated in zinc, wires, and a lemon. Video shows how to measure the amount of voltage produced by battery using a volt meter. [6:47]
Other
Beautiful Chemistry: Beautiful Reactions
Check out a collection of short videos demonstrating a variety of chemical reactions and the apparatus that aids in creating the reactions. Examples of the reactions shared are precipitation and bubbling.
Sophia Learning
Sophia: Define Reaction Rate
This lesson will define reaction rate as the change in concentration of products (molarity) in a chemical reaction over time.
Sophia Learning
Sophia: Definition of a Catalyst
This lesson will define the term catalyst and explain how a catalyst affects the activation energy of a chemical reaction. Examples provided.
Sophia Learning
Sophia: Define Enthalpy
This lesson will define enthalpy and introduce its symbol, providing examples of its use.
Bozeman Science
Bozeman Science: The Rate Limiting Step
In this video Paul Andersen explains why the slowest elementary step in a chemical reaction is the rate-limiting step. This step can be used to determine the overall rate law of the chemical reaction. Take a look at the concept map and...
Bozeman Science
Bozeman Science: Multistep Reactions
Paul Andersen explains how an overall chemical reaction is made up of several elementary steps. The stoichiometry of this equation can be predicted but the rate law must be measured. If the elementary steps of the reaction are determined...
Bozeman Science
Bozeman Science: The Reactions Path
In this video Paul Andersen explains how the reaction path can be described in an energy profile. Enough energy must be added to reach the activation energy required and stress the bonds. Eventually the bonds break and new bonds are...
Bozeman Science
Bozeman Science: Elementary Reactions
In this video Paul Andersen explains that elementary reactions are steps within a larger reaction mechanism. Colliding molecules require sufficient energy and proper orientation to break bonds and form new bonds. A unimolecular reaction...
Bozeman Science
Bozeman Science: The Rate Constant
In this video Paul Andersen describes the characteristics of the rate constant in chemical reactions. The rate constant is highly variable in reactions and must be determined experimentally. The rate constant is dependent on both...
Bozeman Science
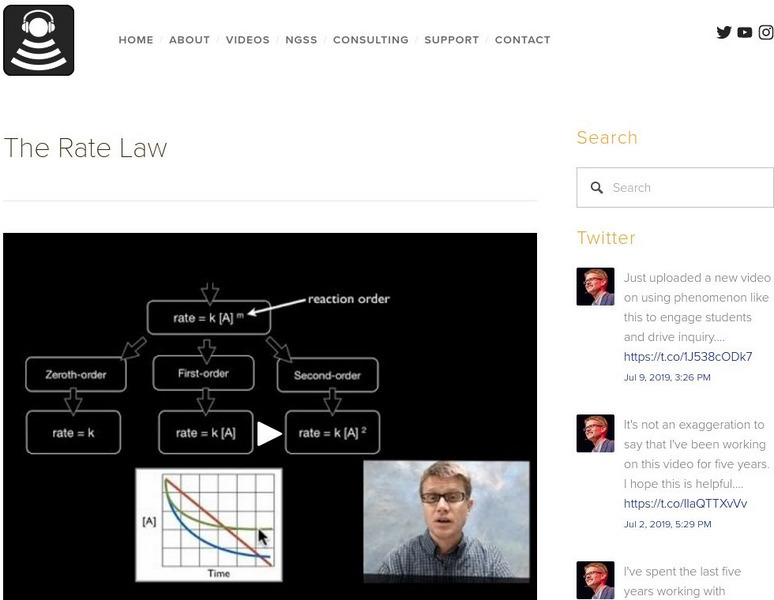
Bozeman Science: The Rate Law
Paul Andersen explains how the rate law can be used to determined the speed of a reaction over time. Zeroth-order, first-order and second-order reactions are described as well as the overall rate law of a reaction. The rate of a reaction...
Sophia Learning
Sophia: Chemical Equation: Vocabulary: Lesson 1
This lesson will explain the general format of a chemical equation including products, reactants, arrow notation, and states of matter. It is 1 of 2 in the series titled "Chemical Equation: Vocabulary."
Sophia Learning
Sophia: Chemical Reactions: Lesson 3
This lesson introduces the process of writing the equation for chemical reactions. It is 3 of 9 in the series titled "Chemical Reactions."
Sophia Learning
Sophia: Chemical Reaction Types: Combustion: Lesson 1
This lesson will show that a combustion reaction is a hydrocarbon burned in oxygen to form water and carbon dioxide. It is 1 of 2 in the series titled "Chemical Reaction Types: Combustion."
Sophia Learning
Sophia: Chemical Reaction Types: Decomposition: Lesson 2
This lesson will demonstrate how to identify and complete a decomposition reaction. It is 2 of 2 in the series titled "Chemical Reaction Types: Decomposition."
Sophia Learning
Sophia: Chemical Reaction Types: Single Replacement: Lesson 1
This lesson will demonstrate how to identify and complete a single replacement reaction. It is 1 of 2 in the series titled "Chemical Reaction Types: Single Replacement."
Crash Course
Crash Course Chemistry #8: Acid Base Reactions in Solution
Today, Hank talks about the actual reactions happening in solutions - atoms reorganizing themselves to create whole new substances in the processes that make our world the one we know and love. This week, we focus on acids and bases and...
Crash Course
Crash Course Chemistry #6: Stoichiometry
Chemists need stoichiometry to make the scale of chemistry more understandable - Hank is here to explain why, and to teach us how to use it. [12:48]
Khan Academy
Khan Academy: Chemistry: Hess's Law and Reaction Enthalpy Change
A video lecture giving an explanation of Hess's law, the law of heat summation. Understand that whether the process takes places in one or several steps the heat released or absorbed in a chemical process is the same. The video also...
Khan Academy
Khan Academy: Chemistry
A video lecture that builds on previous knowledge of enthalpy to find out how much heat energy is either gained or lost in a reaction. By being able to predict how much heat it takes to produce certain products, it is explained to...
Khan Academy
Khan Academy: Synthesis of Alcohols: Preparation of Alcohols Using Li Al H4
Khan Academy explores the use of lithium aluminum hydride to prepare alcohols. [11:17]