Hi, what do you want to do?
University of Colorado
University of Colorado: Physics 2000: Spectral Lines
Several pages from an excellent site which describe the science of spectroscopy. The unique atomic emission (and absorption) line spectrum of elements are illustrated and explained. Includes a Java applet depicting the quantum energy...
PBS
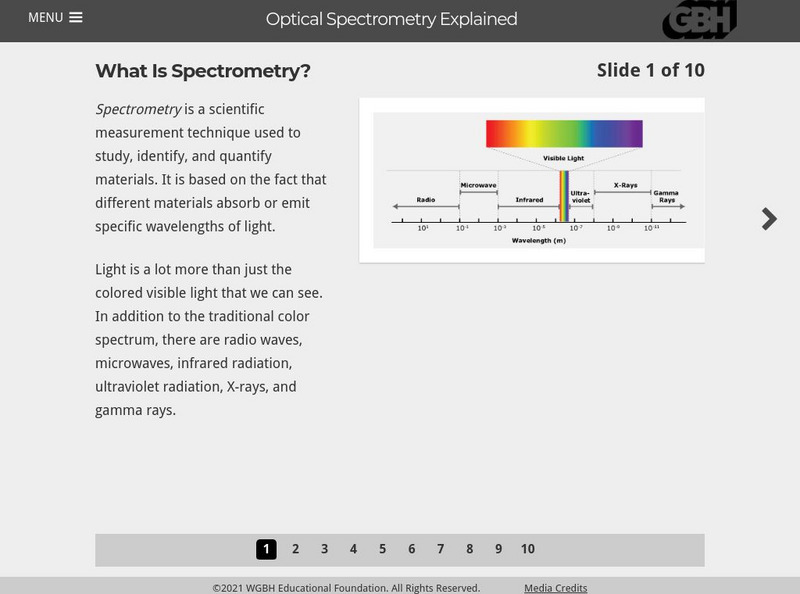
Pbs Learning Media: Spectrometry Explained
This interactive tutorial illustrates how spectrometry is used to study, identify, and quantify materials.
Georgia State University
Georgia State University: Hyper Physics: Hydrogen Energies and Spectrum
This site from Georgia State University gives information on the transitions of electrons between energy levels. The energy levels for electrons in the hydrogen atom are discussed. The Rydberg equation is stated and electron transitions...
University of Colorado
University of Colorado: Physics 2000: Balmer's Formula
A short description of a physics lab involving the determination of the wavelength of the four spectral lines in the hydrogen emission spectrum. Perhaps the most important part of the page is the picture of the spectrometer.
Friesian School
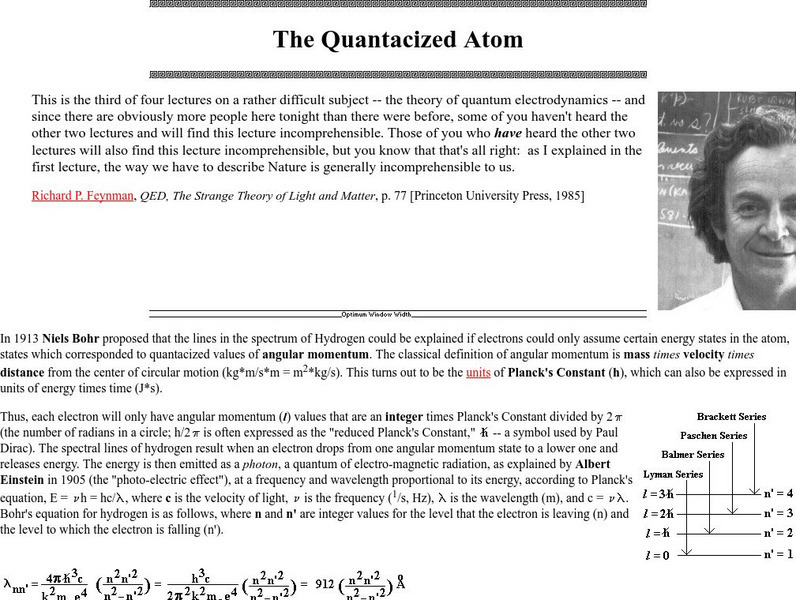
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
McGraw Hill
Mc Graw Hill Companies: Origins of the Quantum Theory
An indexing page for Chapter 27 (Origins of the Quantum Theory) of the companion web site for McGraw Hill's Contemporary College Physics textbook. Includes several worthy pages with chapter notes, simple definitions, online computer...
Simon Fraser University
Chem1 Virtual Textbook: Emission and Absorption Spectra
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site specifically discusses emission and absorption spectra in relation to the Bohr model.
Other
Brockport High School: Energy Levels of Hydrogen Atom
From the Brockport High School Physics Labs web pages. Includes an excellent graphic depicting the energy levels of a hydrogen atoms and portraying the electron level transitions for the Lyman, Balmer, and Paschen series. Includes both...
University of St. Andrews (UK)
University of St. Andrews: Johann Jakob Balmer
Describes the life and scientific contributions of Johann Jakob Balmer. Discussion focuses on Balmer's contributions to the line spectra of hydrogen.