Bozeman Science
Conservation of Atoms
In this video Paul Andersen explains how atoms are conserved in a chemical reaction. This can be seen in a chemical equation where the subscripts represent the atoms in the molecule and the coefficients represent the molecules. The mass...
Bozeman Science
Thinking in Matter - Level 4 - Conservation of Matter
In this video Paul Andersen shows conceptual thinking in a mini-lesson on the conservation of matter. TERMS Matter - physical substances Atoms - the basic unit of elements Conservation - the quantity of a physical quantity remains...
Wonderscape
Understanding the Law of Conservation of Matter
Explore the concept of the conservation of matter, which states that during a chemical reaction, matter is neither created nor destroyed. Learn how this fundamental principle applies to various examples, from dissolving sugar in tea to...
Professor Dave Explains
The Law of Conservation of Matter
When you eat a burrito, where does all that matter go? What about when matter changes phases, does any matter appear or disappear? When we talk about this sort of stuff, reality sometimes goes against our intuition, so we'd better make...
Wonderscape
Science Kids: All About Chemical Reactions
This video is an educational program about physical and chemical changes in matter. The host uses examples such as baking cookies and a rusty bike fender to explain the concepts of physical properties of matter, chemical reactions,...
Massachusetts Institute of Technology
Mit: Blossoms: Recognizing Chemical Reactions
An interactive video lesson where students first determine misconceptions about what happens during chemical reactions, and then investigate some various chemical reactions.
Bozeman Science
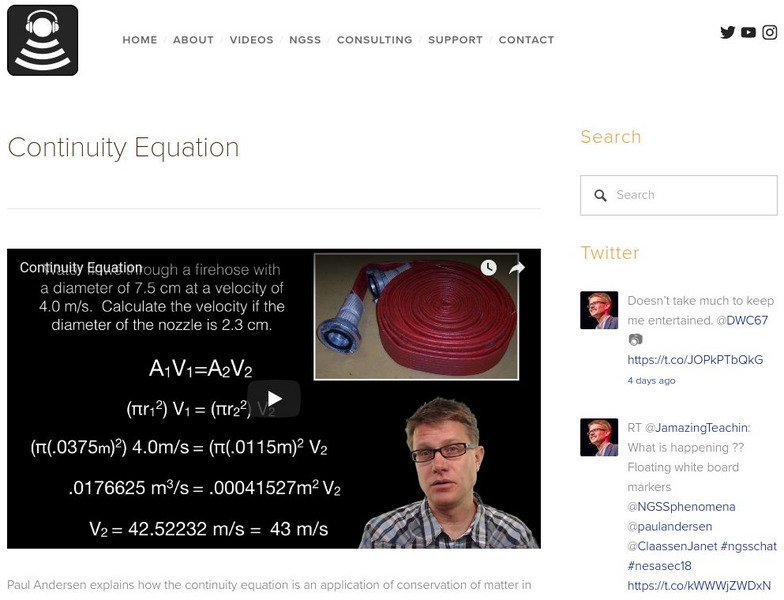
Bozeman Science: Continuity Equation
Paul Andersen explains how the continuity equation is an application of conservation of matter in a fluid. The continuity equation may apply to either mass or volumetric flow. Example problem and examples are included. [4:05]