National Institute of Open Schooling
General Characteristics of the p-Block Elements
The 20th installment in a series of 36 focuses on the characteristics of the p-block elements. Learners discuss, read about, and answer questions pertaining to the occurrence of these elements in nature, their electron configurations,...
Science Teachers
Basic Periodic Table of the Elements
This basic, black-and-white printable is a copy of the periodic table of elements. It includes the standard element name, symbol, atomic number, and atomic weight. Across the bottom is an explanation that says for elements without stable...
National Institute of Open Schooling
Periodic Table and Atomic Properties
An in-depth lesson, the fourth activity in a series of 36, begins with teaching how the periodic table's arrangement came to its current design. Using this knowledge, pupils then move on to analyze the arrangement of elements to their...
Chapman University
The Standard Model Poster
Chemistry classes will appreciate this color-coded, single-page reference sheet for The Standard Model of particle physics. It is divided into two main sections: elementary particles and compound particles, both with their antiparticles....
Texas Education Agency
Texas Gateway: Ap Physics I: Appendices: Atomic Masses
This is a chart of the elements including the atomic mass, atomic number, symbol, half-life, and more.
Web Elements
Web Elements Periodic Table: Boron
A great deal of information on boron, and its physical and chemical properties, all arranged and organized in a very convenient way.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: The Pauli Exclusion Principle
The Pauli Exclusion Principle shows how electrons fill atomic orbitals. Includes biographical information on Wolfgang Pauli.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: Electron Clouds and Energy Levels
An explanation of the different types of atomic orbitals, how they are filled according to the Pauli Exclusion Principle, and how many electrons can fit in each electron shell.
Chemicool
Chemi Cool: Vanadium
Set of tables describing many physical and chemical properties of the element vanadium.
Atomic Archive
Atomic Archive: Nuclear Fusion
From the Atomic Archive - the online companion to the award-winning CD-ROM. This page defines nuclear fusion and depicts the process by an informative diagram. Includes numerical values which describe the typical energy values for fusion...
Wikimedia
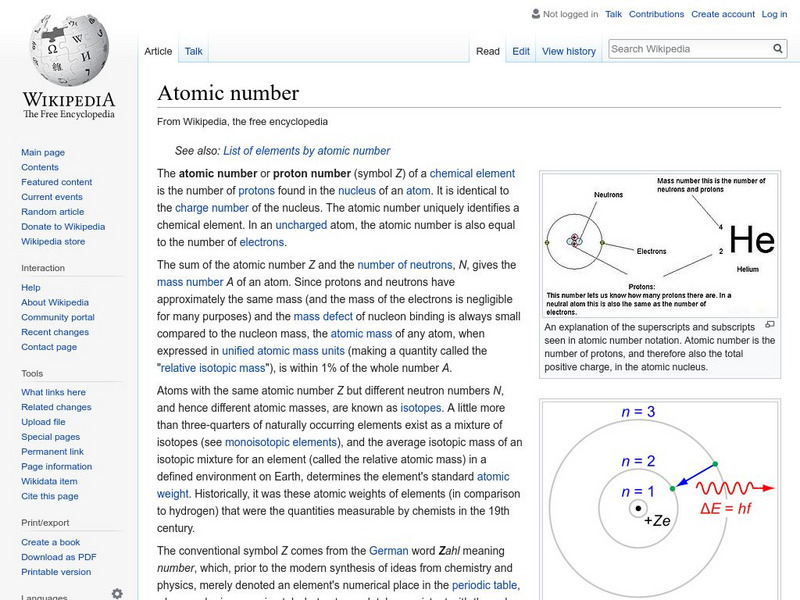
Wikipedia: Atomic Number
Wikipedia provides the definition of the term, "Atomic number," a term used in chemistry and physics to represent the number of protons in the nucleus of an atom.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: Quantum Numbers
Each electron has a set of quantum numbers that specify it's location, orbital, and energy in a unique manner.
Encyclopedia of Earth
Encyclopedia of Earth: Physics & Chemistry: Calcium
Information about the element, Calcium, atomic number 20. Covers physical and atomic properties, sources, its role in geochemical cycles, how abundant it is on the Earth, its isotopes, some compounds of calcium, and its importance to...
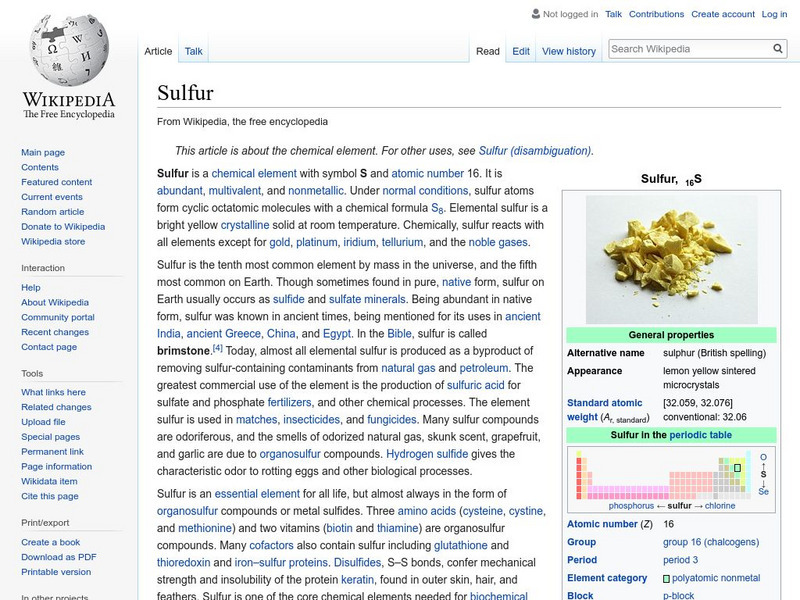
Wikimedia
Wikipedia: Sulfur
This site from the Wikipedia Encyclopedia provides information on atomic properties, physical properties and notable characteristics of sulfur can be found on this informative site. Links are provided for additional information.
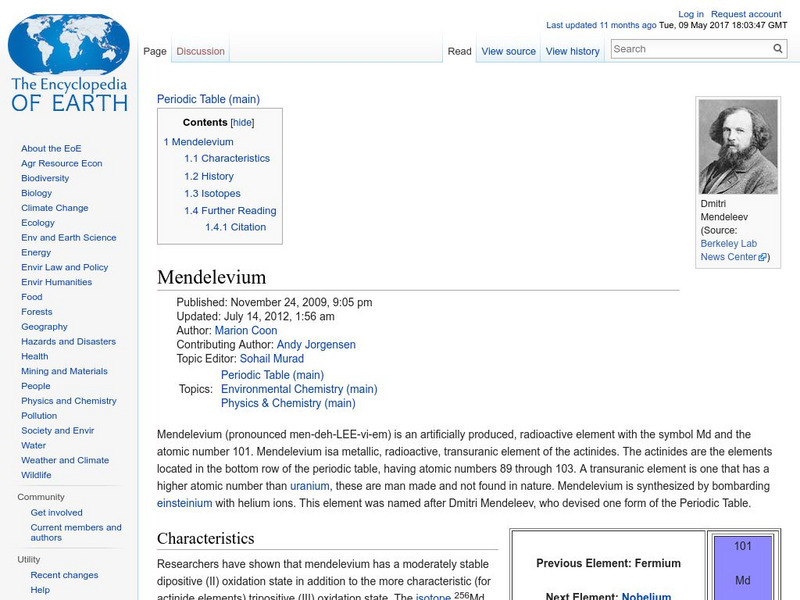
Encyclopedia of Earth
Encyclopedia of Earth: Mendelevium
Information about the synthetic, radioactive element, Mendelevium, atomic number 101. Covers its discovery, physical and atomic properties, and details about permissible exposure.
Encyclopedia of Earth
Encyclopedia of Earth: Uranium
Information about the radioactive element, Uranium, atomic number 92. Describes its history, physical and atomic properties, how abundant it is on the Earth, and permissible exposure limits. Also discusses sources, uses, isotopes,...
Encyclopedia of Earth
Encyclopedia of Earth: Rhenium
Information about the metallic element, Rhenium, atomic number 75. Covers its physical and atomic properties, how abundant it is on the Earth, sources, uses, and potential substitutes.
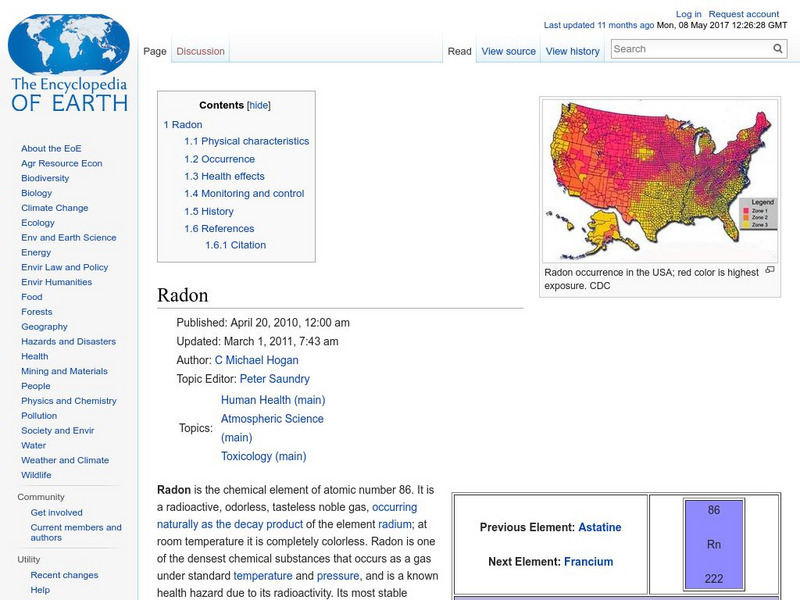
Encyclopedia of Earth
Encyclopedia of Earth: Radon
Information about the radioactive element, Radon, atomic number 86. Covers physical properties, atomic properties, how abundant it is on the Earth, details about its impact on human health, and about how it can be monitored.
Encyclopedia of Earth
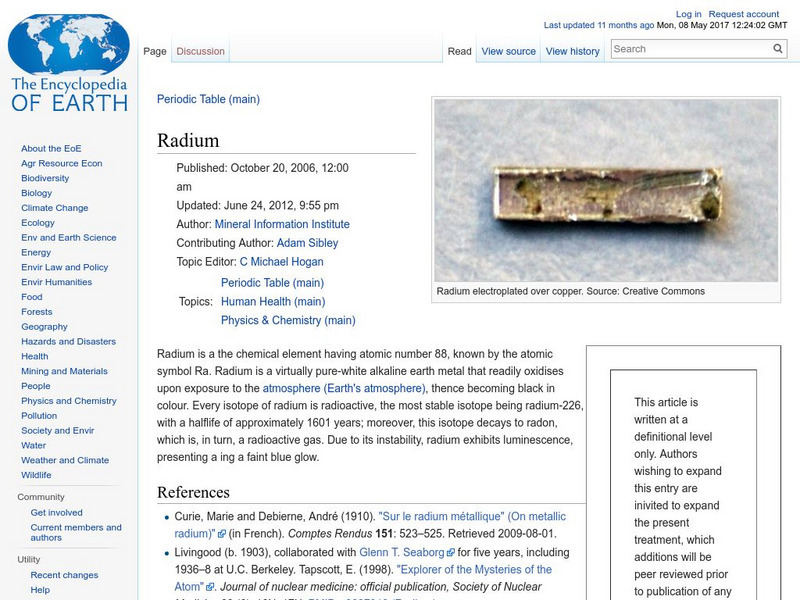
Encyclopedia of Earth: Radium
Information about the radioactive element, Radium, atomic number 88. Covers physical and atomic properties, how abundant it is on the Earth, and permissible exposure limits.
Encyclopedia of Earth
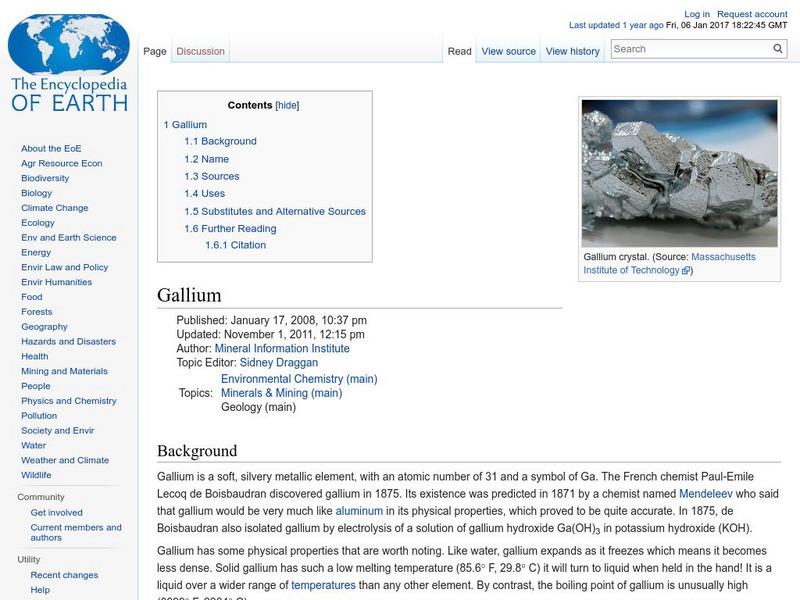
Encyclopedia of Earth: Gallium
Information about the metallic element, Gallium, atomic number 31. Covers its discovery, its physical and atomic properties, sources, uses, how abundant it is on the Earth, and permissible exposure limits.
Encyclopedia of Earth
Encyclopedia of Earth: Chromium
Information about the element, Chromium, atomic number 24. Covers discovery, physical and atomic properties, how abundant it is on the Earth, permissible exposure limits, sources, uses, and potential substitutes.
Encyclopedia of Earth
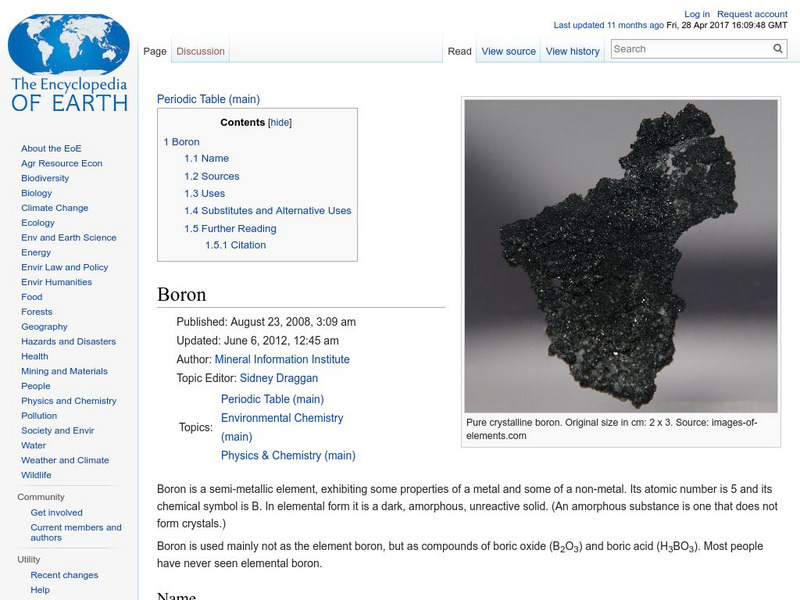
Encyclopedia of Earth: Boron
Information about the semi-metallic element, Boron, atomic number 5. Covers physical and atomic properties, how abundant it is on the Earth, sources, uses, and possible substitutes.
Encyclopedia of Earth
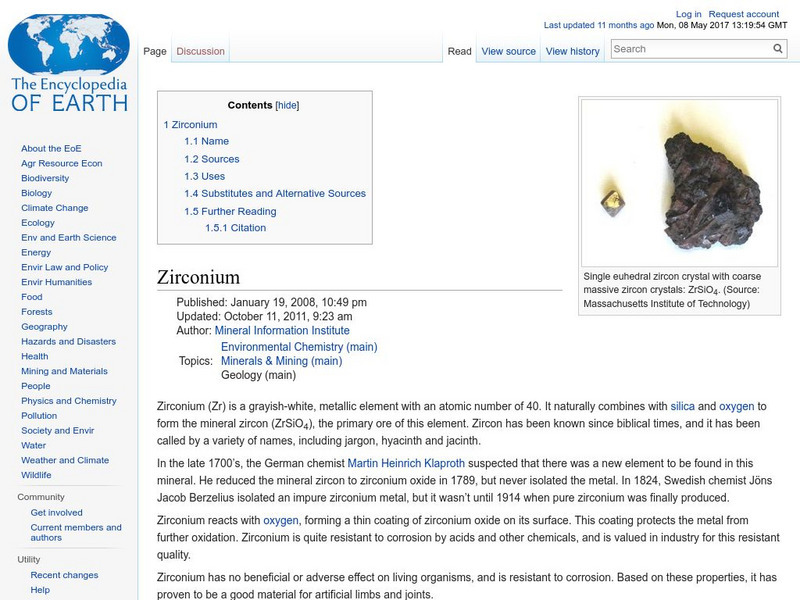
Encyclopedia of Earth: Zirconium
Information about the element Zirconium, atomic number 40. Covers history of its discovery, sources, physical properties, atomic properties, how abundant it is on the Earth, details about permissible exposure, uses, and possible...
Encyclopedia of Earth
Encyclopedia of Earth: Ytterbium
Information about the element, Ytterbium, atomic number 70. Covers physical properties, atomic properties, how abundant it is on the Earth, and details about health-related regulations.