Hi, what do you want to do?
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Oxidation Reduction Reactions [Pdf]
Read about how oxidation-reductions affect our everyday lives. View how atoms, molecules, and ions are reduced during the oxidation-reduction process. Learn how some of the normal chemical reactions in the body can change DNA. Use the...
OpenStax
Open Stax: Andrew R. Barron: Valence Shell Electron Pair Repulsion (Vsepr) Theory
Detailed explanation of Valence Shell Electron Pair Repulsion (VSEPR) Theory with examples and questions for the reader to check comprehension. Also includes step-by-step instructions for correctly building molecules using VSEPR Theory....
Curated OER
Science Kids: Science Images: Chemical Structure of Methane
This diagram shows the chemical structure of methane. A methane molecule contains one carbon atom and four hydrogen atoms.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Molecular Compounds: Audio Book
Learn all about molecular structures in this easy-to-understand, audio book. See how valence electrons determine how atoms bond together, and look at the bonding inside some of the most common molecules around you.
Smithsonian Institution
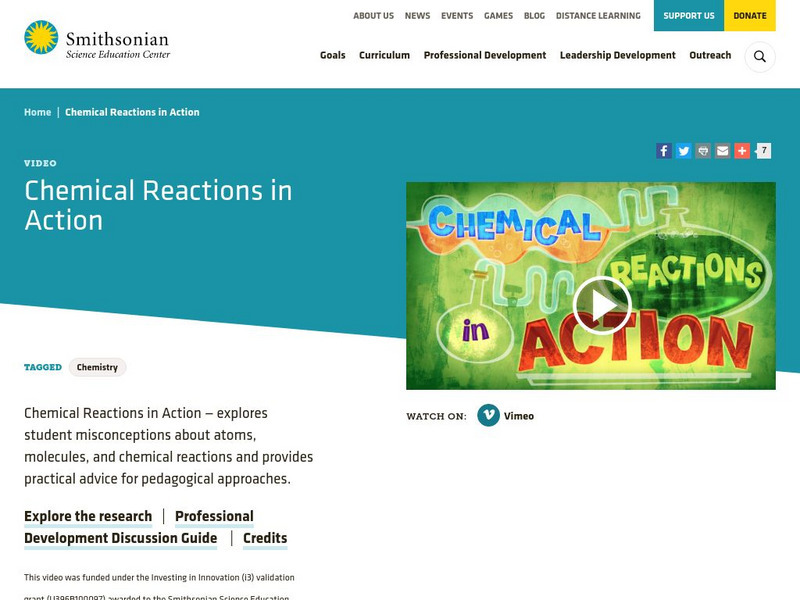
Smithsonian Science Education Center: Chemical Reactions in Action
This video is a professional development resource that explores student misconceptions about atoms, molecules, and chemical reactions. It also gives some pedagogical approaches for approaching these subjects. [10:12]
University Corporation for Atmospheric Research
Ucar: Hydrocarbons
Learn about "hydrocarbons", chemical compounds whose molecules are made up entirely of carbon and hydrogen atoms.
Texas Instruments
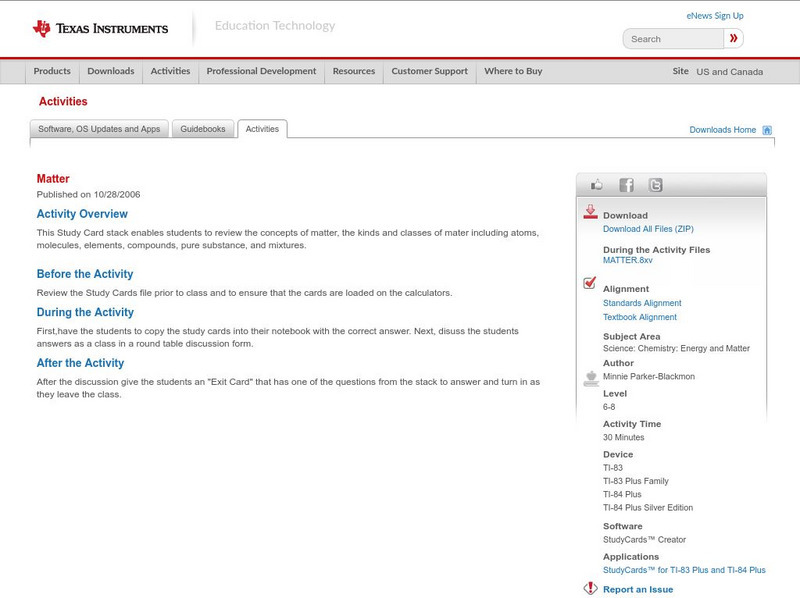
Texas Instruments: Matter
This Study Card stack enables students to review the concepts of matter, the kinds and classes of mater including atoms, molecules, elements, compounds, pure substance, and mixtures.
McREL International
Mc Rel: Whelmer #33 Learning Activity: Density Balloon
A simple activity that investigates the effect of heat on the volume of a gas. The activity is presented in lesson plan format that meets NSES standards.
Other popular searches
- Gumdrop Atoms and Molecules
- Making Atoms and Molecules
- Science, Atoms and Molecules
- Science Atoms and Molecules
- Atoms and Molecules Lesson
- Atoms and Molecules Musical
- Combining Atoms and Molecules
- Molecules and Atoms
- Atoms Molecules and Element
- Courting Atoms and Molecules
- Elements Molecules and Atoms





![Chiral Publishing: An Introduction to Chemistry: Oxidation Reduction Reactions [Pdf] eBook Chiral Publishing: An Introduction to Chemistry: Oxidation Reduction Reactions [Pdf] eBook](https://static.lp.lexp.cloud/images/attachment_defaults/resource/large/FPO-knovation.png)