Hi, what do you want to do?
TED Talks
Ted: Ted Ed: What Is the Heisenberg Uncertainty Principle?
The Heisenberg Uncertainty Principle states that you can never simultaneously know the exact position and the exact speed of an object. Why not? Because everything in the universe behaves like both a particle and a wave at the same time....
National High Magnetic Field Laboratory
Magnet Academy: Low Temperature Physics
Why do physicists want to study things at temperatures so cold atomic motion almost comes to a halt? And how do they create such frigid environments, anyway? Read on for the what, how and why of low temperature physics.
Nobel Media AB
The Nobel Prize: The Quantised World
After an introduction, this site breaks down into sections discussion quantum theory, including A Quantum Theory for Energy, A Quantum Theory for Atomic Structures, Waves or Particles, Quantum Mechanics, and Interpreting the Quantum World.
Georgia State University
Georgia State University: Hyper Physics: Wave Particle Duality
The dualistic nature of light is discussed. The photoelectric effect and the Davisson-Germer experiment are contrasted as empirical evidence supporting each of the two views - particle and wave - of the nature of light. The photoelectric...
Famous Scientists
Famous Scientists: Arnold Sommerfeld
Learn about the scientist who pioneered developments in atomic and quantum physics, and also served as PhD supervisor for many Nobel Prize winners in physics and chemistry.
Famous Scientists
Famous Scientists: Hans Bethe
Find out about Hans Bethe, one of the founders of quantum physics.
Famous Scientists
Famous Scientists: Wolfgang Ernst Pauli
Learn about the life and work of one of the pioneers of quantum physics, Wolfgang Ernst Pauli.
Massachusetts Institute of Technology
Mit: Open Course Ware: Courses: Physics: String Theory for Undergraduates
College-level physics course focusing on the string theory. The course develops the aspects of string theory and makes it accessible to students familiar with basic electromagnetism and statistical mechanics. Course features include...
Cosmo Learning
Cosmo Learning: Modern Physics: Quantum Mechanics
A collection of video lectures from a modern physics course that focuses on quantum mechanics. The course is taught at Stanford University. The course includes ten lectures that vary in length.
Wolfram Research
Wolfram Science World: Quantum Electrodynamics
In its description of quantum electrodynamics this definition provides the governing equations.
Simon Fraser University
Chem1 Virtual Textbook: The Quantum Atom
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site specifically addresses the quantum atom and related topics. The related topics include the wave function and its physical...
Georgia State University
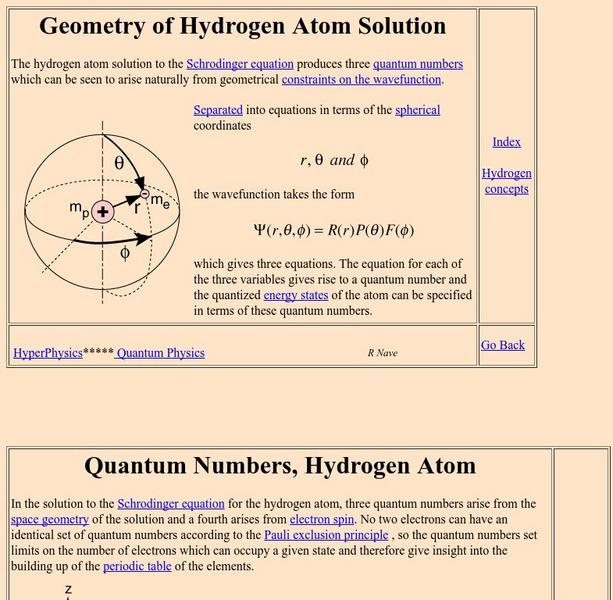
Georgia State University: Hyper Physics: Quantum Numbers, Hydrogen Atom
This tutorial contains links to explanations of the four different quantum numbers (principal, orbital, magnetic, and spin). Equations for each are provided.
Libre Text
Libre Texts: Physics: Early Quantum Mechanics
An overview of quantum mechanics which begins with why they are important and wraps up with an accident at Bell Telephone labs in 1925. Find several theories and formulas.
University of Colorado
University of Colorado: Physics 2000: Quantum Atom
Several pages with an interesting discussion of the visible light spectrum and atomic absorption and emission line spectrum. Features excellent graphics, thorough and understandable discussion, and many interactive Java applets.
Ohio State University
Betha Chemistry Tutorial
This site resource offers tutorials on the gas laws, balancing chemical equations, and quantum mechanics.
Chemistry Collective
Chem Collective: Statistical Mechanical Simulator
The Statistical Mechanics Simulator allows students to create a multi-level quantum mechanical system and explore the partition function and thermodynamic properties.
Massachusetts Institute of Technology
Mit: Open Course Ware: Courses: Physics: Classical Mechanics
College-level online course highlighting the study of classical mechanics. This course focuses on Newtonian mechanics, fluid mechanics, and kinetic gas theory. Course features include a 35 video lecture series by Walter Lewin. Also link...
Lawrence Berkeley National Laboratory
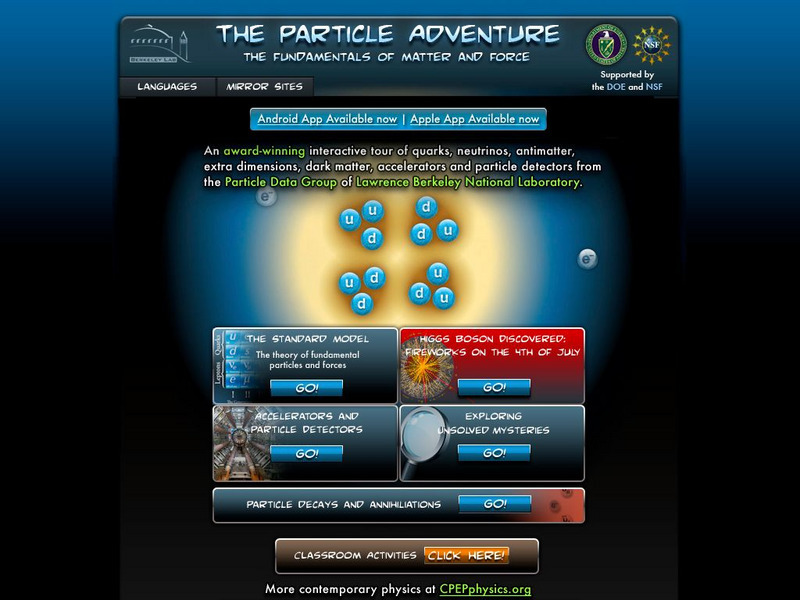
Berkeley Lab: The Particle Adventure
Visit this site for an interactive tour of the atom and all aspects of particle physics. View the animations available with almost every description on this site. A great place for the fundamentals of particles and forces including a...
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: The Pauli Exclusion Principle
The Pauli Exclusion Principle shows how electrons fill atomic orbitals. Includes biographical information on Wolfgang Pauli.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: Electron Clouds and Energy Levels
An explanation of the different types of atomic orbitals, how they are filled according to the Pauli Exclusion Principle, and how many electrons can fit in each electron shell.
American Association of Physics Teachers
Com Padre Digital Library: Open Source Physics: Rectangular Well Superposition
A simulation that displays the 2D evolution of the position-space of a wave in an infinite 2D rectangular well.
Nobel Media AB
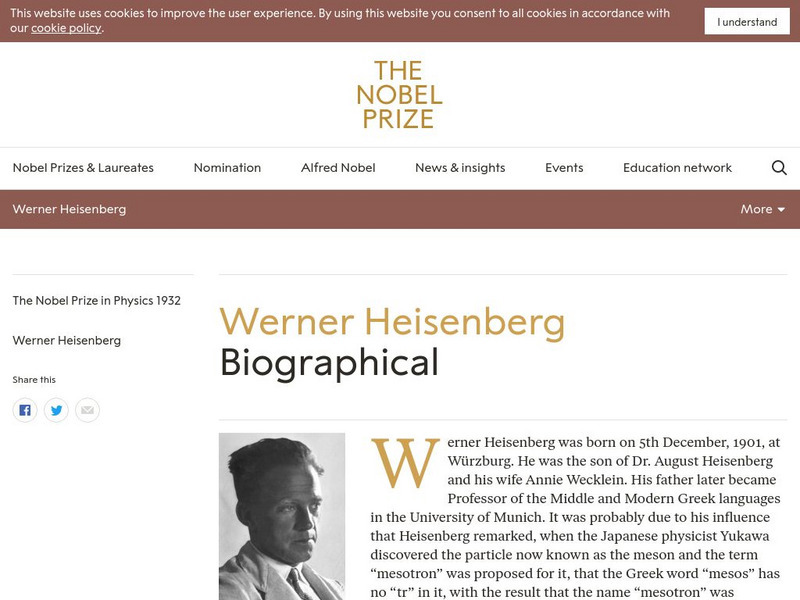
The Nobel Prize: Werner Karl Heisenberg Biographical
This website provides information on the life and scientific contributions of Werner Heisenburg, a recipient of the Nobel Prize in Physics for his "creation of quantum mechanics." Read the Prize Presentation Speech in which Professor H....
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: Spin
A basic explanation of spin and how it relates to electrons and quantum numbers.
Other
The International Linear Collider: Gateway to the Quantum Universe [Pdf]
This fascinating report describes the planned International Linear Collider, a particle accelerator, and explains how it will work and the scientific rationale behind it. Much of the material is presented in relatively easy to understand...

















![The International Linear Collider: Gateway to the Quantum Universe [Pdf] Handout The International Linear Collider: Gateway to the Quantum Universe [Pdf] Handout](http://lessonplanet.com/content/resources/thumbnails/410018/large/bwluav9tywdpy2symdiwmduymc0ymzuwmy0xeg4wzg1tlmpwzw.jpg?1589984780)