Wyzant
Wyzant: Chem Tutor: Gases
A lengthy page covering most all the gas laws. Each law is described in words and stated as an equation. The use of each law in solving problems is demonstrated. Some problem-solving tips are provided. There are 23 practice problems with...
CK-12 Foundation
Ck 12: Stoichiometric Calculations
[Free Registration/Login may be required to access all resource tools.] Based on the balanced chemical equation, students will calculate the masses or moles of reactants or products generated in a given reaction. They will also convert...
Science Education Resource Center at Carleton College
Serc: Using an M&m Magma Chamber to Illustrate Magmatic Differentiation
In this lesson students will understand the process of magmatic differentiation by fractional crystallization using a simple experiment.
CK-12 Foundation
Ck 12: Algebra: Dimensional Analysis
[Free Registration/Login may be required to access all resource tools.] Learn how to solve problems using dimensional analysis.
CK-12 Foundation
Ck 12: Plix Series: Gas Density: Mass, Volume and the Mole
[Free Registration/Login Required] Find out what mathematical function to perform when converting between particles and moles. Then answer one challenge question about the topic.
CK-12 Foundation
Ck 12: Plix Series: Limiting Reactant
[Free Registration/Login Required] Using atoms from a reactants pool, construct products of an equation shown and place them in the product pool. Then answer a challenge question about the topic.
Sophia Learning
Sophia: Converting From Mass to Particles Using a Balanced Chemical Equation
A narrated screencast explaining how to use a balanced chemical equation to determine the number of particles of a substance given the starting mass of another substance. [4:46]
Sophia Learning
Sophia: Define Reversible Reaction
Find out what a reversible reaction is in this narrated screencast. Also learn how this is different from a reaction that comes to completion. [2:48]
Sophia Learning
Sophia: Limiting Reactant Percent Yield
A video lesson which explains how to determine the percent yield of a substance using the theoretical yield of the chemical reaction. [5:13]
Sophia Learning
Sophia: Limiting Reactant Theoretical Yield
Find out the procedure in determining the theoretical yield of a product in a chemical reaction. [6:25]
Chem4kids
Chem4 Kids: Reactions
This site provides a detailed overview of chemical reactions. Content explores what a reaction is, as well as different types of reactions, reaction rates, how to measure compounds to create a reaction, and "stuff" you can add to a...
CK-12 Foundation
Ck 12: Algebra: Dimensional Analysis
[Free Registration/Login may be required to access all resource tools.] Learn how to solve problems using dimensional analysis.
Frostburg State University
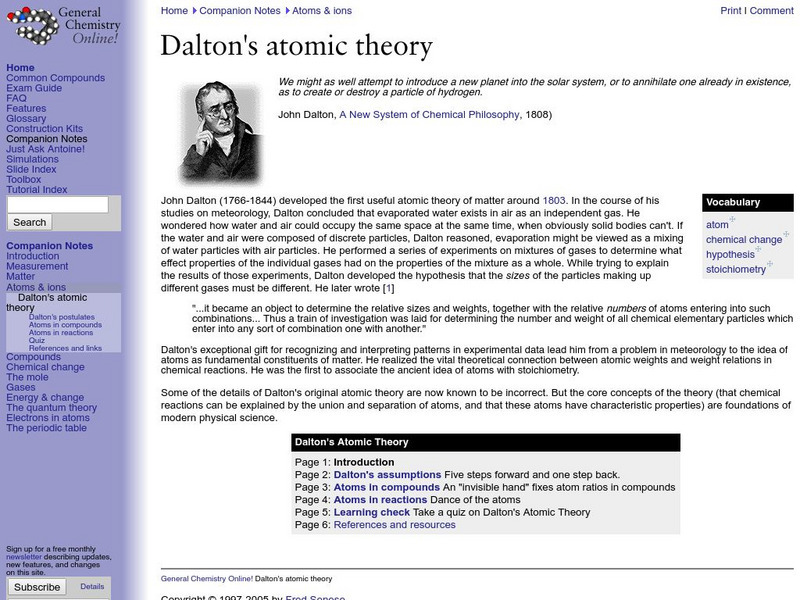
General Chemistry Online: Dalton's Atomic Theory
Discusses John Dalton's development of the atomic theory and the four main points of his theory. Includes references and a link to a quiz on Dalton's Atomic theory.
CK-12 Foundation
Ck 12: Chemistry for High School
This digital textbook covers core chemistry concepts and includes interactive features, real-world examples, and videos.
Khan Academy
Khan Academy: Single Replacement Reactions
Definition of single replacement (or single displacement) reactions. Predicting and determining the products using the reactivity series.
Chem Tutor
Chem Tutor: Solutions
An in-depth look at solutions and their properties. Includes practice problems for calculating solution concentration.
Sophia Learning
Sophia: Limiting Reactant Percent Yield: Lesson 1
This lesson will demonstrate how to determine the percent yield of a substance using the theoretical yield of the reaction. It is 1 of 2 in the series titled "Limiting Reactant - Percent Yield."
Other popular searches
- Stoichiometry Worksheets
- Moles and Stoichiometry
- Chemistry Stoichiometry
- Stoichiometry Labs
- Percent Yield Stoichiometry
- Gas Law Stoichiometry
- Stoichiometry Lesson Plan
- Stoichiometry Volume
- Equilibrium Stoichiometry
- Candy Stoichiometry
- Gas Stoichiometry
- Acid Base Stoichiometry