CK-12 Foundation
Ck 12: Chemistry: Solute and Solvent
[Free Registration/Login may be required to access all resource tools.] Covers solutes and solvents.
John Wiley & Sons
Wiley Higher Education: Strong Acid & Base Dissociation
This page has animated, 3-D graphics that illustrate the dissociation of strong acids and strong bases and the formation of aqueous solutions.
Mocomi & Anibrain Digital Technologies
Mocomi: Difference Between Solution Solute and Solvent
Article offers a comparison between a solution, a solute, and a solution and provides examples.
ClassFlow
Class Flow: Water & Aqueous Systems
[Free Registration/Login Required] This flipchart provides a multitude of hands-on activities for identifying, arranging and creating sentence maps for various water and aqueous systems.
Texas Instruments
Texas Instruments: Soluble or Not?
This StudyCard activity allows students to apply solubility rules to aqueous solutions. Students then determine whether the compounds are soluble or not in aqueous solution.
Sophia Learning
Sophia: Definition of Dissociation
Find out how ionic compounds dissociate into positive and negative ions in an aqueous solution. [1:59]
Utah Education Network
Uen: Forming Ionic Compounds
Students will practice making and recording observations about various compounds and their aqueous solutions, write the formulas for the compounds, then perform chemical reactions and write the names and formulas of the products.
National Institutes of Health
Ncbi: Amino Acids Are Linked by Peptide Bonds to Form Polypeptide Chains
Proteins are linear polymers formed by linking the a-carboxyl group of one amino acid to the a-amino group of another amino acid with a peptide bond. The formation of a dipeptide from two amino acids is accompanied by the loss of a water...
CK-12 Foundation
Ck 12: Electrolysis
[Free Registration/Login may be required to access all resource tools.] Students investigate voltaic and electrolytic cells describing the reactions that occur during the electrolysis of water. They also identify the products that would...
Other
What Is Life: Aqueous Solutions
Discussion of water as a solvent. Includes material on hydrogen bonds, hydration, hydrophobic effect, acids, basses, and pH.
CK-12 Foundation
Ck 12: Solubility Equilibrium
[Free Registration/Login may be required to access all resource tools.] Students write the solubility product constant expressions for nearly insoluble ionic compounds and perform calculations for compounds and solubility.
Annenberg Foundation
Annenberg Learner: Virtual Particle Lab: Dissolving
Explore what happens when one substance dissolves into another. Run the simulations and see if you can predict the results.
Libre Text
Libre Text: Precipitation Reactions
Precipitation Reactions occur when cations and anions of aqueous solutions combine to form an insoluble ionic solid, called a precipitate. Whether or not such a reaction occurs can be determined by using the solubility rules for common...
Science Buddies
Science Buddies: Solubility of Proteins
Some proteins are soluble in aqueous solutions and some are not. Insoluble proteins can be a problem because the proteins can form large aggregates in solution which are difficult to purify, crystallize, and use in experiments. Compare...
Texas Instruments
Texas Instruments: Naming and Writing Formulas of Acids
This StudyCard activity will enable students to practice naming and writing formulas for binary and non-binary inorganic acids. This activity will also allow students to practice naming acids found in aqueous solution as well as those...
John Wiley & Sons
Wiley: Dissociation of Strong and Weak Electrolytes
This page has animated 3-D graphics that illustrate the dissociation of strong and weak electrolytes.
McMaster University
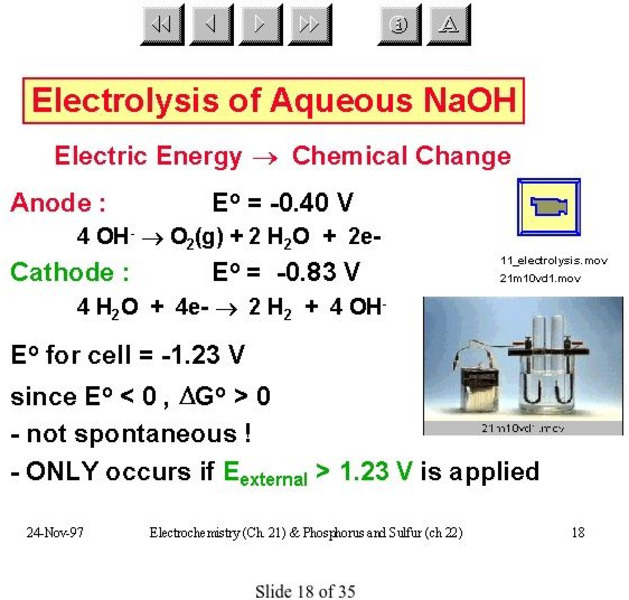
Mc Master University: Electrolysis of Aqueous Na Oh
Experimental setup and energy requirements for electrolysis of sodium hydroxide solution.
Science Education Resource Center at Carleton College
Serc: Do Water Molecules Have Space Between Them?
In this chemistry lab, students investigate whether water molecules have any space between them by filling a glass with water, and adding salt without the water overflowing. They will also experiment with the temperature of the water.