Hi, what do you want to do?
PBS
Pbs: Scientific American Frontiers: Teaching Guide: Tasty Models (9 12)
In this hands-on instructional activity, students use candy to construct models of nutrients (carbohydrates, proteins, and fats). Create visualizations that will aid in learning about dehydration synthesis and bond saturation in fat...
CK-12 Foundation
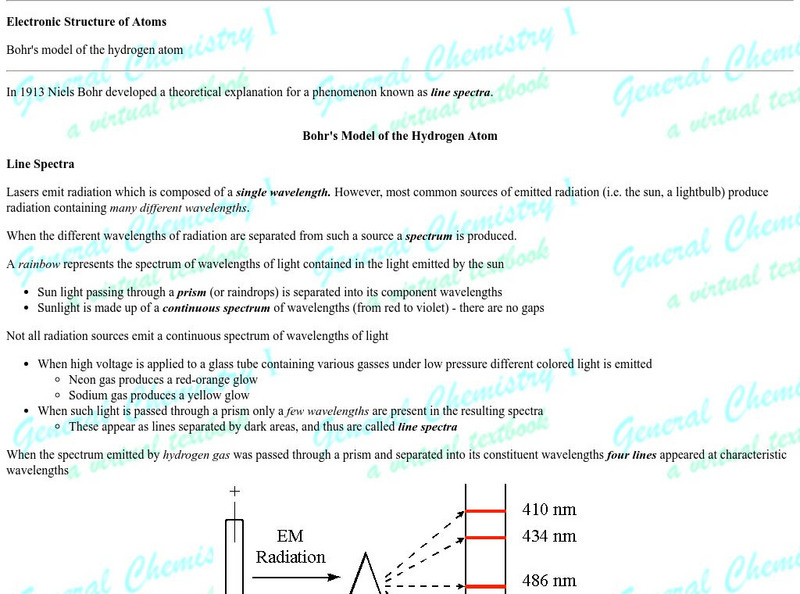
Ck 12: The Bohr and Quantum Models of the Atom
[Free Registration/Login may be required to access all resource tools.] Students explore how the study of the hydrogen emission spectrum led to the Bohr model of the atom, in which electrons exist in states of constant energy.
Science Struck
Science Struck: Explanation of John Dalton's Atomic Theory
Explains the basic tenets of Dalton's atomic theory.
Science Struck
Science Struck: Plum Pudding Model
Describes J.J. Thomson's theory of atomic structure, called the 'Plum Pudding Model,' and how it was disproved by Ernest Rutherford.
CK-12 Foundation
Ck 12: Modern Atomic Theory
[Free Registration/Login may be required to access all resource tools.] Rutherford's model of the atom was better than earlier models. But it wasn't the last word. Danish physicist Niels Bohr created a more accurate and useful model....
Concord Consortium
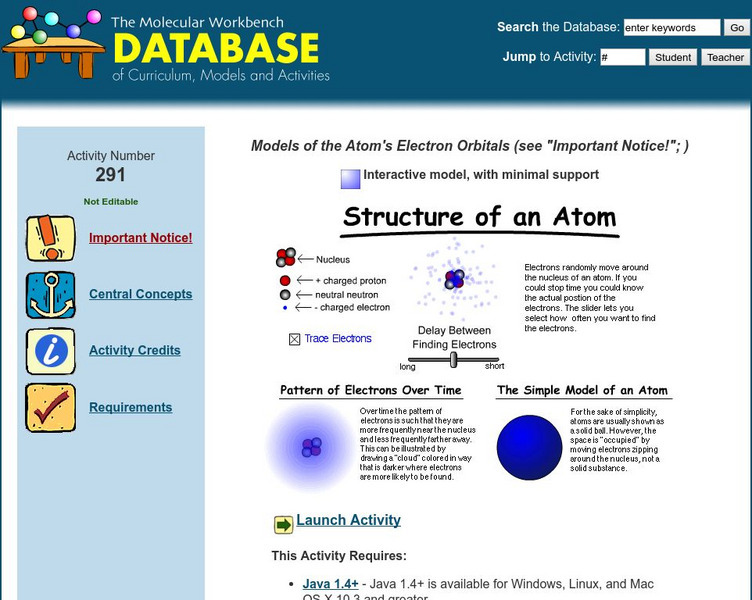
The Molecular Workbench Database: Models of the Atom's Electron Orbitals
Learn about atomic structure and the multiple theories of atomic structure in this simulation.
Michael Blaber, PhD
Florida State University: The Bohr Model of the Atom
A well designed clear tutorial explaining the energies involved in the Bohr model of the atom. Illustrations add to the clearly presented equations.
Google
Google for Education: Model Electron Configuration Using Computational Thinking
With this demonstration, students will learn to use computational thinking to better understand how the atomic number of an element affects how its electrons are configured.
ClassFlow
Class Flow: Atomic Model and Orbital Quiz
[Free Registration/Login Required] This flipchart contains a 20 point multiple choice quiz on atomic history and modeling.
Science Struck
Science Struck: James Chadwick's Atomic Theory and Its Lasting Impact
Explains how James Chadwick came to develop his atomic theory by building upon the work of other scientists.
CK-12 Foundation
Ck 12: Chemistry Simulation: Rutherford's Gold Foil Experiment
[Free Registration/Login Required] How can we predict an atom's structure, if we cannot see an atom? Using the Rutherford's Gold Foil Experiment, make your own model and test out the model.
Sophia Learning
Sophia: Atomic Mass: Lesson 4
This lesson explains what is represented by the atomic mass, and how it varies from one element to the next. Module includes a slideshow and a quiz.
Science Struck
Science Struck: Electron Cloud Model
Explains what the electron model is and the scientists who contributed to its development.
The Wonder of Science
The Wonder of Science: Ms Ps1 1: Atomic Composition Model
A collection of lesson plans for helping students understand atomic composition. Site uses work samples, phenomena, assessment templates, and videos to plan lessons to describe the atomic composition of simple molecules.
Tom Richey
Slide Share: Atomic Structure
Slideshow looking at the history of models of the atom, including those proposed by John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick. Discusses subatomic particles, including the numbers of protons, neutrons,...
CK-12 Foundation
Ck 12: Modern Atomic Theory
[Free Registration/Login may be required to access all resource tools.] Rutherford's model of the atom was better than earlier models. But it wasn't the last word. Danish physicist Niels Bohr created a more accurate and useful model....
Science Education Resource Center at Carleton College
Serc: Investigating Isotopes: Using M&m's as a Model for Calculating Atomic Mass
This activity allows young scholars to gain an understanding of the relationship between the mass and relative abundance of an isotope and its effect on the average atomic mass of the element. Students will be given a random sample of...
CK-12 Foundation
Ck 12: The Bohr Model of the Atom
[Free Registration/Login may be required to access all resource tools.] Students will learn about the history of atomic theory, and the development of the Bohr model of the atom. Includes a simulation for exploring the Bohr Model.
Upper Canada District School Board
Tom Stretton's Advanced Placement Chemistry: Atomic Structure and Periodicity
This chemistry e-textbook provides students with AP-level reading and practice material on atomic structure and periodicity.
Other
Cronodon: Atoms Models of the Atom
Presents models of the atom developed by Thomson, Rutherford, Bohr, and Schrodinger. Includes successes and failures of each, illustrations, and detailed descriptions. Also discusses Dirac's model of the atom and introduces quantum...
BBC
Bbc: Gcse Bitesize: Atomic Structure
This lesson focuses on early ideas about the structure of an atom including John Dalton's plum pudding model and Ernest Rutherford's nuclear model. A link to a test is provided.
Lawrence Berkeley National Laboratory
Berkeley Lab: Particle Adventure: The Standard Model
An introduction to the Standard Model, a theory which attempts to explain atomic structure using leptons, quarks, and force carrier particles.
Science Education Resource Center at Carleton College
Serc: Quantum Atomic Structure
This multi-day lesson plan helps students to understand how the models of the atom have changed and how quantum mechanics affects the electrons as they orbit the nucleus.
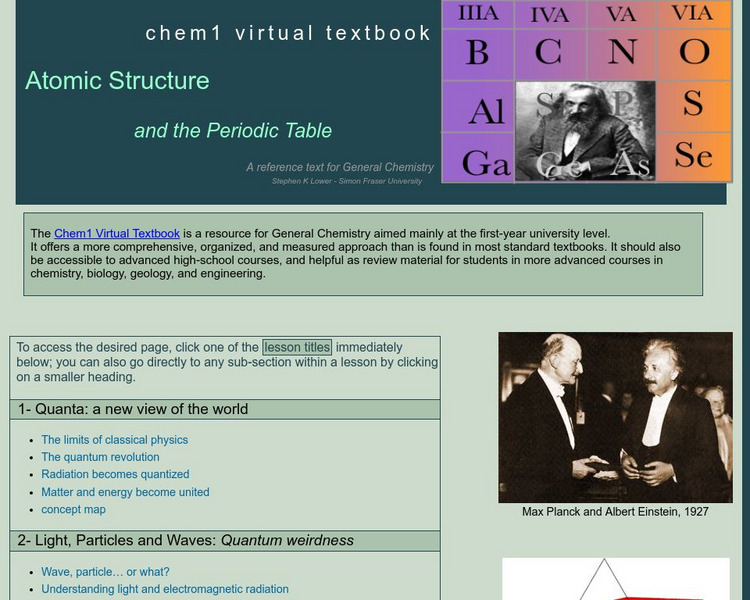
Simon Fraser University
Chem1 Virtual Textbook: Atoms and the Periodic Table
As part of the General Chemistry Virtual Textbook, this site examines a list of related topics (in hyperlink format) on the atom and the Periodic Table of elements. Topics from light, particles, and waves to the Bohr model, quantum...
Other popular searches
- Bohr Atomic Model
- History of Atomic Model
- Edible Atomic Model
- Atomic Model History
- Atomic Model Chlorine
- Illustrate All Atomic Model
- Atomic Model for Chlorine
- 3d Atomic Model
- Atomic Model Discovery
- Atomic Models Discovery
- Atomic Models Spectra
- Modern Atomic Model