Hi, what do you want to do?
Science Education Resource Center at Carleton College
Serc: Modeling Valence Electrons
In this activity, students use a map of electron configuration and bingo chips to configure electrons for elements that are given on a problem card. Students will see patterns of elements and their valence electrons in relation to the...
Simon Fraser University
Chem1 Virtual Textbook: The Shell Model of the Atom
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses the properties of the atoms individually in relation to the main group elements of the Periodic Table.
Vision Learning
Visionlearning: Atomic and Ionic Structure of First 12 Elements
Analyze the electron configuration of the of the first twelve elements on the Periodic Table.
National High Magnetic Field Laboratory
Magnet Academy: Joseph John Thomson
Joseph John Thomson, better known as J. J. Thomson, was a British physicist who first theorized and offered experimental evidence that the atom was a divisible entity rather than the basic unit of matter, as was widely believed at the...
Science Struck
Science Struck: The Structure of an Atom: A Labeled Diagram
Looks at the scientists who developed the model of the atom by building on previous scientists' discoveries about its structure.
Texas Instruments
Texas Instruments: The History Behind the Atom
This StudyCard activity enables students to review the key contributions by scientists and philosophers towards are current understanding of the atom. It also allows students to review characteristics of the major atomic models.
Khan Academy
Khan Academy: Discovery of the Electron and Nucleus
Learn about Thomson's cathode ray experiment and the plum pudding model and Rutherford's gold foil experiment.
Concord Consortium
Concord Consortium: Molecular Workbench Showcase: Chemistry, Chemical Bonds
Simulations to show students the nature of chemical bonds. Simulations include an explanation of stereochemistry, atomic orbitals, chemical polarity, formation of an atom, and a summary quiz.
Concord Consortium
Concord Consortium: Molecular Workbench: Atomic Orbitals
View the approximate locations of electrons in valence shells 1s through 4f.
Science Education Resource Center at Carleton College
Serc: Electron Energy Levels of Atoms and Ions
In this lab, young scholars investigate basic electron structure by making a model using pennies and different sized filter papers.
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Rutherford Scattering
How did Rutherford figure out the structure of the atom without being able to see it? Simulate the famous experiment in which he disproved the Plum Pudding model of the atom by observing alpha particles bouncing off atoms and determining...
PBS
Nova: The Atom Builder
A brief explanation is provided for designing a stable atom. You can also refer to a labeled model of a carbon atom. This resource also has a link to an atom building activity.
Other
Chemtopics: Development of Modern Atomic Theory [Pdf]
A summary of the achievements of J. J. Thomson, Ernest Rutherford, Niels Bohr, and Erwin Schrodinger.
Boise State University
Boise State University: Atoms: A Virtual Field Trip Through Time and Space
Learn about the models of the atom that have been proposed throughout history. Presents Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and the modern theory of the atom. Sections are accompanied by journal...
Wisc-Online
Wisc Online: Lewis Dot Structures of Covalent Compounds
Short slide show provides basic information about drawing Lewis dot structures for covalent compounds. Starts with anatomy of the atom, and then shows the relationship between atomic particles and the Periodic Table of Elements. Offers...
Simon Fraser University
Chem1 Virtual Textbook: Bohr's Theory
Acting as part of an overview on quantum theory, this section of the site answers the question, "How did Bohr's theory save the planetary model, for a while?" A section below also discusses the primary problems with Bohr's theory.
Other
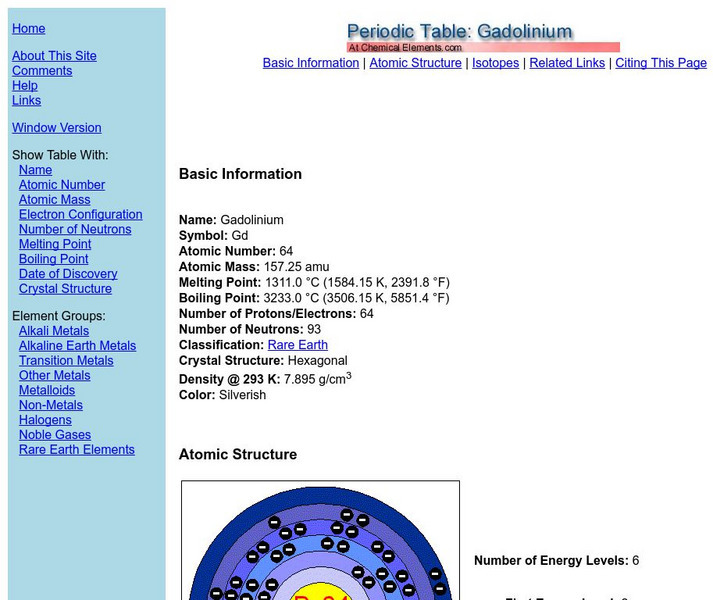
Chemical Element: Periodic Table: Gadolinium
Basic properties of the element Gadolinium Includes Bohr model.
CK-12 Foundation
Ck 12: Structure of the Atom
[Free Registration/Login may be required to access all resource tools.] Students learn about the important discoveries of subatomic particles, and how they led to our current understanding of the atom.
Lawrence Berkeley National Laboratory
Berkeley Lab: The Atom
Presented is an overview of atomic theory concentrating on the experiments of Ernest Rutherford.
CK-12 Foundation
Ck 12: Chemistry Simulation: Neon Lights
[Free Registration/Login Required] Neon lights are a type of discharge tube. Observe how electrons create colored light in a hydrogen gas discharge tube. Can you figure out why hydrogen's emission spectrum contains more than one color of...
PBS
Pbs: People & Discoveries Niels Bohr
A wonderful PBS biography of a great man. This contains many personal touches, yet does a fine job of describing Bohr's scientific work. But it's best at Bohr the man. Nice quotes, one in the middle, one at the end.
CK-12 Foundation
Ck 12: Flex Book Textbooks: Chemistry Second Edition
[Free Registration/Login may be required to access all resource tools.] A complete, web-based, multi-media textbook covering a wide variety of Chemistry concepts.
Thomas Jefferson National Accelerator Facility
Jefferson Lab: It's Elemental Periodic Table of Elements
This page displays the Periodic Table of Elements and allows you to click each individual element for a page that displays in-depth information about that particular element. There are also links to many different games and learning...
National High Magnetic Field Laboratory
Magnet Academy: Enrico Fermi
Enrico Fermi was a titan of twentieth-century physics. He outlined the statistical laws that govern the behavior of particles that abide by the Pauli exclusion principle and developed a theoretical model of the atom in his mid-twenties....
Other popular searches
- Bohr Atomic Model
- History of Atomic Model
- Edible Atomic Model
- Atomic Model History
- Atomic Model Chlorine
- Illustrate All Atomic Model
- Atomic Model for Chlorine
- 3d Atomic Model
- Atomic Model Discovery
- Atomic Models Discovery
- Atomic Models Spectra
- Modern Atomic Model