Center for Applied Linguistics
Chemical Interactions: Atoms and Bonding
Watch budding chemists interact with the resource on chemical interactions. In the unit, six lessons provide an overview of basic chemistry, from understanding the development of atomic theory to distinguishing between ionic and covalent...
Columbus City Schools
To Measure its Mass or Volume?
Atoms, elements, and molecules, oh my! Teaching the fundamentals of chemistry to curious sixth graders has never been easier to accomplish. Here is a resource that pulls together everything needed to get them off to a good start,...
Royal Society of Chemistry
Colour—Gifted and Talented Chemistry
Add a splash of color to your chemistry class! Science scholars discover the principles behind color through a wide variety of hands-on activities. Lessons include dyes, chromatography, and flame tests.
Curated OER
Quantum Mechanics and Atomic Physics
Students will discuss the de Brogile Hypothesis and state the circumstances under which the wave nature of matter is observed. They will also calculate the wavelengths of matter waves.
Curated OER
Quantum Physics
Students discuss the mass-energy relationship based on Einstein's work. They calculate the energy released in various scenerios and sketch diagrams for the Lyman, Balmer and Pfund Series. In groups, they discuss the role of photons and...
CK-12 Foundation
Ck 12: Chemistry: Gold Foil Experiment: Rutherford's Atomic Model
[Free Registration/Login may be required to access all resource tools.] This site explains Rutherford's discovery of the nucleus and the planetary model of the atom and the role of gold foil experiment in refining the atomic model. It...
CK-12 Foundation
Ck 12: Physical Science: Electron Cloud Atomic Model
[Free Registration/Login may be required to access all resource tools.] Features the electron cloud atomic model and atomic orbitals.
CK-12 Foundation
Ck 12: Chemistry: Thomson's Atomic Model
[Free Registration/Login may be required to access all resource tools.] Explains the idea of a scientific model and the development of Thomson's plum pudding model of the atom.
CK-12 Foundation
Ck 12: Physical Science: Thomson's Atomic Model
[Free Registration/Login may be required to access all resource tools.] Thomson's discovery of the electron and his plum pudding model of the atom.
CK-12 Foundation
Ck 12: Physical Science: Rutherford's Atomic Model
[Free Registration/Login may be required to access all resource tools.] Rutherford's discovery of the nucleus and his planetary model of the atom.
Vision Learning
Visionlearning: Atomic Theory and Structure: The Early Days
Explanation of the evolution of atomic theory and the experimentation associated with these concepts.
CK-12 Foundation
Ck 12: Atomic Theory
[Free Registration/Login may be required to access all resource tools.] In this online tutorial students will explain the law of conservation of mass, the law of definite proportions, and the law of multiple proportions. They will also...
CK-12 Foundation
Ck 12: Physical Science: Dalton's Atomic Theory
[Free Registration/Login may be required to access all resource tools.] Reintroduction of the idea of the atom and the billiard ball model.
CK-12 Foundation
Ck 12: Chemistry: Bohr's Atomic Model
[Free Registration/Login may be required to access all resource tools.] Explains the basic principles of the Bohr hydrogen atom.
CK-12 Foundation
Ck 12: The Nuclear Model of the Atom
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will distinguish between the three main subatomic particles and understand the contributions of J. J. Thomson, Robert...
CK-12 Foundation
Ck 12: Evolution of the Atomic Model
[Free Registration/Login may be required to access all resource tools.] Students take a closer look at how our understanding of the atom has evolved over time.
Khan Academy
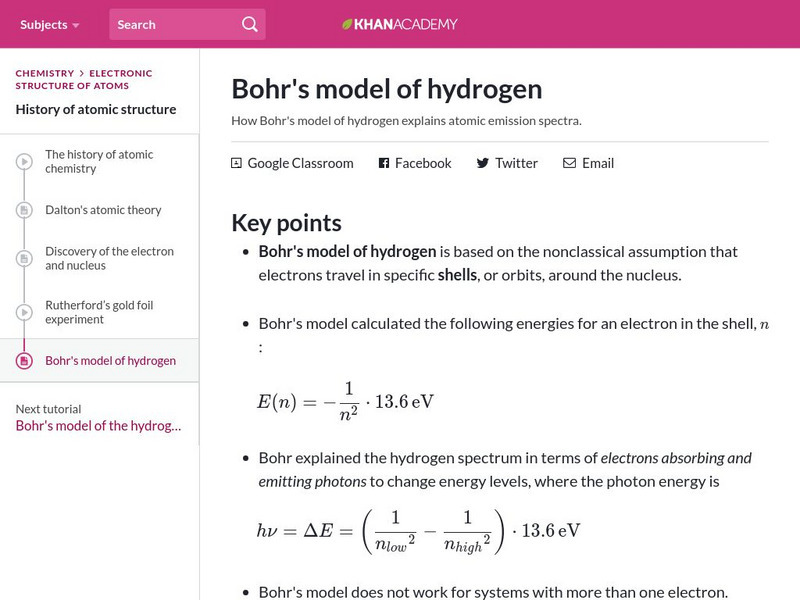
Khan Academy: Bohr's Model of Hydrogen
Resource investigates how Bohr's model of hydrogen explains atomic emission spectra.
Texas Education Agency
Texas Gateway: Atomic Theory: Dalton, Thomson and Rutherford
This tutorial will help students understand how Dalton, Thomson, and Rutherford contributed to the model of the atom.
CK-12 Foundation
Ck 12: The Bohr and Quantum Models of the Atom
[Free Registration/Login may be required to access all resource tools.] Students explore how the study of the hydrogen emission spectrum led to the Bohr model of the atom, in which electrons exist in states of constant energy.
CK-12 Foundation
Ck 12: Modern Atomic Theory
[Free Registration/Login may be required to access all resource tools.] Rutherford's model of the atom was better than earlier models. But it wasn't the last word. Danish physicist Niels Bohr created a more accurate and useful model....
Sophia Learning
Sophia: Atomic Mass: Lesson 4
This lesson explains what is represented by the atomic mass, and how it varies from one element to the next. Module includes a slideshow and a quiz.
CK-12 Foundation
Ck 12: Modern Atomic Theory
[Free Registration/Login may be required to access all resource tools.] Rutherford's model of the atom was better than earlier models. But it wasn't the last word. Danish physicist Niels Bohr created a more accurate and useful model....
CK-12 Foundation
Ck 12: The Bohr Model of the Atom
[Free Registration/Login may be required to access all resource tools.] Students will learn about the history of atomic theory, and the development of the Bohr model of the atom. Includes a simulation for exploring the Bohr Model.
BBC
Bbc: Gcse Bitesize: Atomic Structure
This lesson focuses on early ideas about the structure of an atom including John Dalton's plum pudding model and Ernest Rutherford's nuclear model. A link to a test is provided.
Other popular searches
- Bohr Atomic Model
- History of Atomic Model
- Edible Atomic Model
- Atomic Model History
- Atomic Model Chlorine
- Illustrate All Atomic Model
- Atomic Model for Chlorine
- 3d Atomic Model
- Atomic Model Discovery
- Atomic Models Discovery
- Atomic Models Spectra
- Modern Atomic Model