Encyclopedia of Earth
Encyclopedia of Earth: Rhenium
Information about the metallic element, Rhenium, atomic number 75. Covers its physical and atomic properties, how abundant it is on the Earth, sources, uses, and potential substitutes.
Encyclopedia of Earth
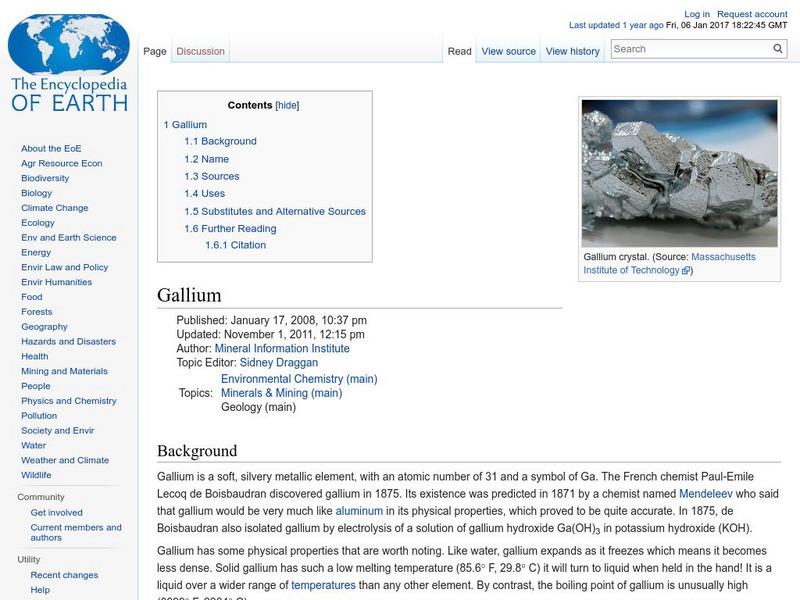
Encyclopedia of Earth: Gallium
Information about the metallic element, Gallium, atomic number 31. Covers its discovery, its physical and atomic properties, sources, uses, how abundant it is on the Earth, and permissible exposure limits.
Encyclopedia of Earth
Encyclopedia of Earth: Chromium
Information about the element, Chromium, atomic number 24. Covers discovery, physical and atomic properties, how abundant it is on the Earth, permissible exposure limits, sources, uses, and potential substitutes.
Encyclopedia of Earth
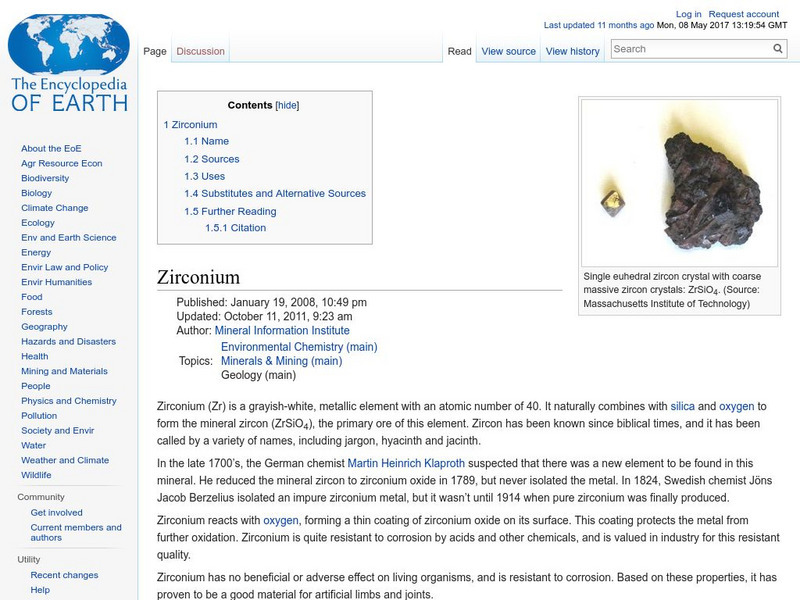
Encyclopedia of Earth: Zirconium
Information about the element Zirconium, atomic number 40. Covers history of its discovery, sources, physical properties, atomic properties, how abundant it is on the Earth, details about permissible exposure, uses, and possible...
Encyclopedia of Earth
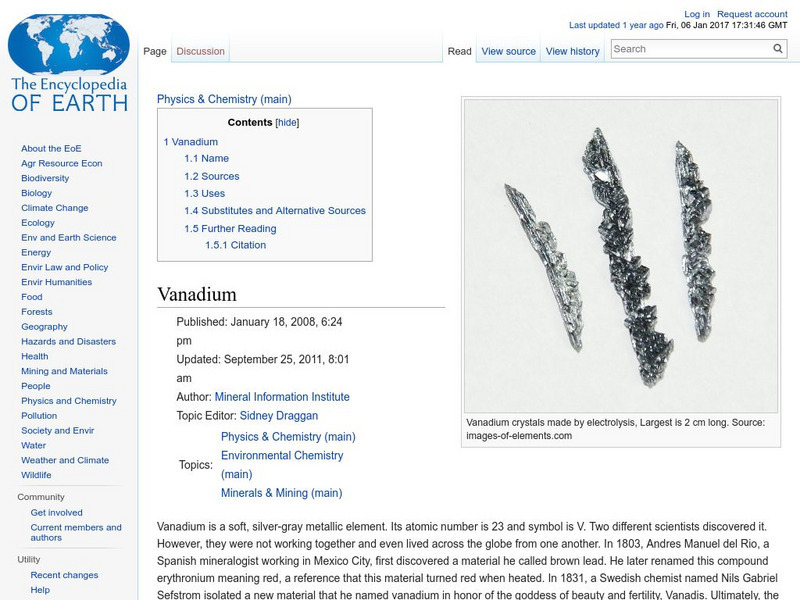
Encyclopedia of Earth: Vanadium
Information about the element, Vanadium, atomic number 23. Covers its history, sources, physical and atomic properties, prevalence on the Earth, details about any health-related regulations, uses, and possible substitutes.
Encyclopedia of Earth
Encyclopedia of Earth: Titanium
Information about the element, Titanium, atomic number 22. Covers its initial discovery, physical properties, atomic properties, how abundant it is on the Earth, and details about health-related regulations. Also discusses sources, uses,...
Encyclopedia of Earth
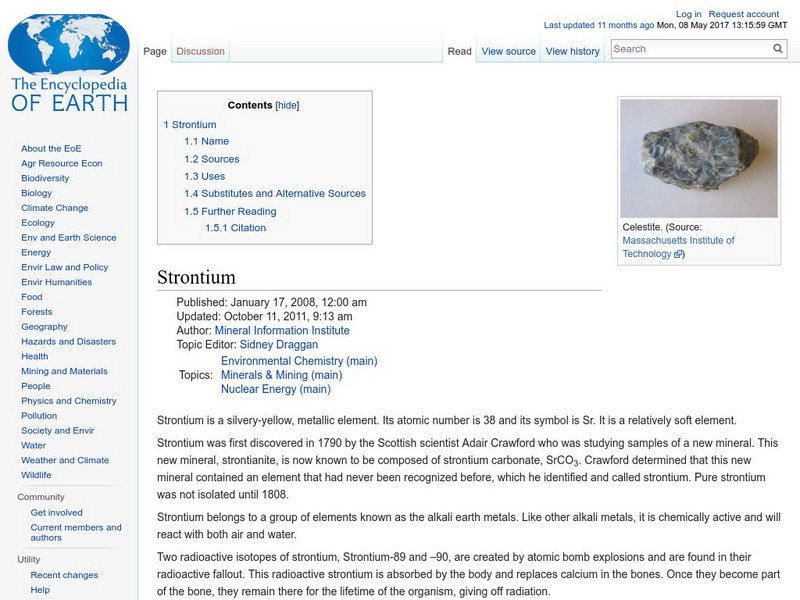
Encyclopedia of Earth: Strontium
Information about the element, Strontium, atomic number 38. Covers its history, sources, physical properties, atomic properties, how abundant it is on the Earth, details about permissible exposure, uses, and possible substitutes.
Encyclopedia of Earth
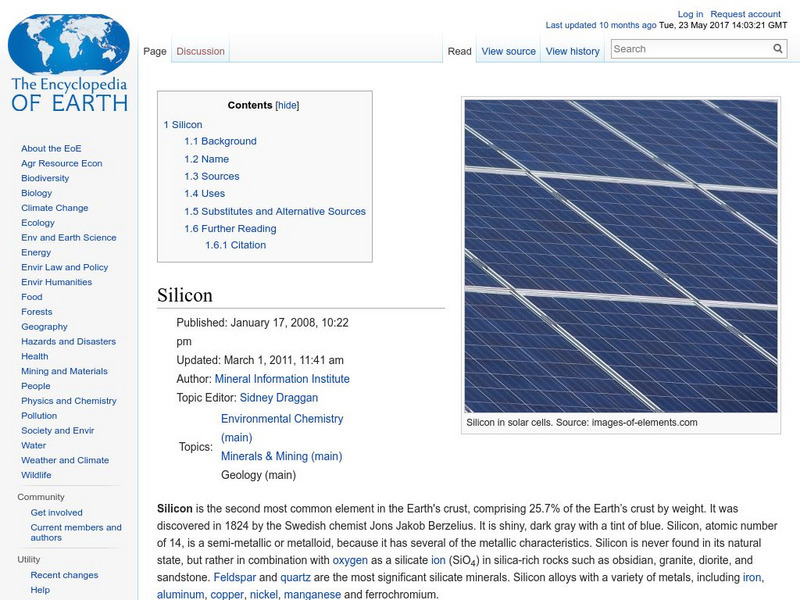
Encyclopedia of Earth: Silicon
Information about the element, Silicon, atomic number 14. Covers physical properties, atomic properties, how abundant it is on the Earth, and details about health-related regulations. Also discusses sources of Silicon, and its many...
Encyclopedia of Earth
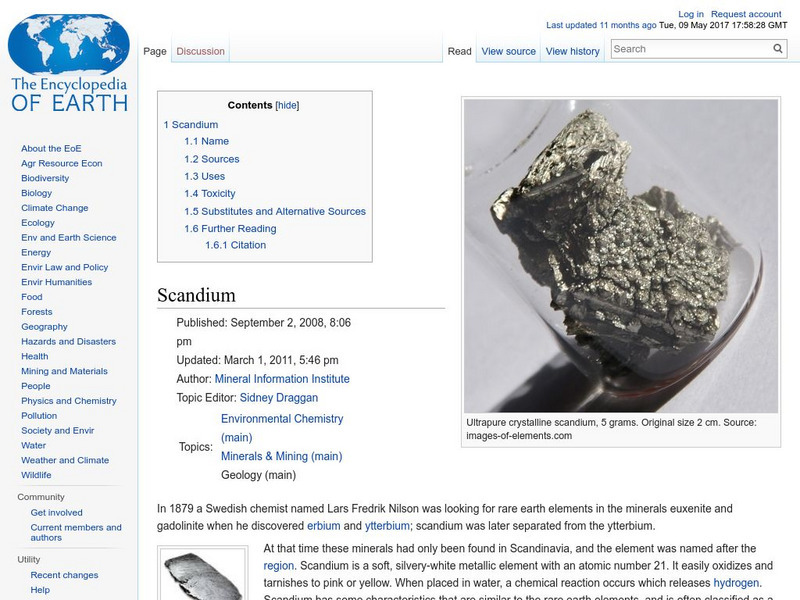
Encyclopedia of Earth: Scandium
Information about the element, Scandium, atomic number 21. Covers physical properties, atomic properties, how abundant it is on the Earth and where it is found, and details about any impact on human health.
Encyclopedia of Earth
Encyclopedia of Earth: Platinum
Information about the element, Platinum, atomic number 78. Covers its physical and atomic properties, how abundant it is on the Earth, sources, uses, potential substitutes, and permissible exposure limits.
Encyclopedia of Earth
Encyclopedia of Earth: Manganese
Information about the element, Manganese, atomic number 25. Covers physical properties, atomic properties, how abundant it is on the Earth, details about health-related regulations, and its importance to human health. Also discusses...
Encyclopedia of Earth
Encyclopedia of Earth: Iron
Information about the element, Iron, atomic number 26. Covers physical properties, atomic properties, how abundant it is on the Earth, and details about health-related regulations, and its importance to plant and to animal health. Also...
Encyclopedia of Earth
Encyclopedia of Earth: Iodine
Information about the element, Iodine, atomic number 53. Covers physical and atomic properties, how abundant it is on the Earth, permissible exposure limits, sources, uses, and potential substitutes.
Encyclopedia of Earth
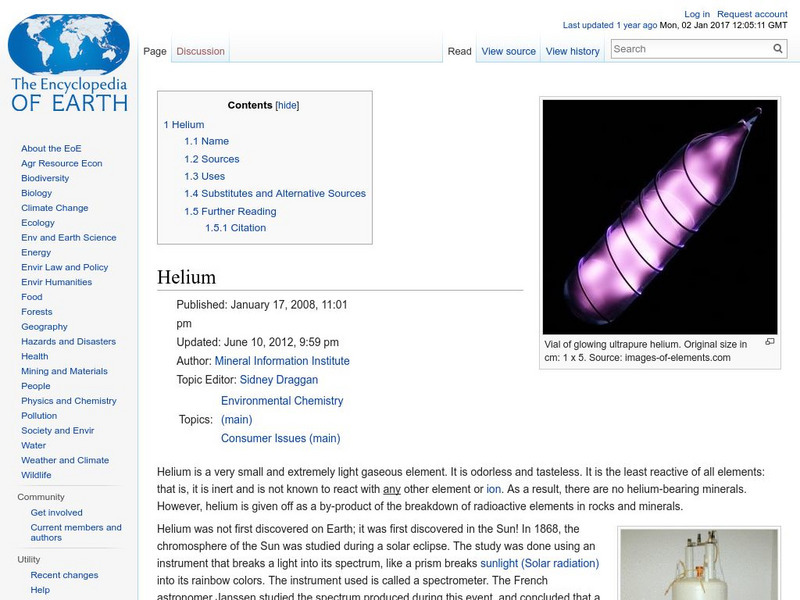
Encyclopedia of Earth: Helium
Information about the element, Helium, atomic number 2. Covers its discovery, physical and atomic properties, how abundant it is on the Earth, its uses, possible substitutes, and permissible exposure limits.
Encyclopedia of Earth
Encyclopedia of Earth: Gold
Information about the element, Gold, atomic number 79. Covers its unique physical properties, its atomic properties, how abundant it is on the Earth, sources, uses, and potential substitutes.
Encyclopedia of Earth
Encyclopedia of Earth: Cobalt
Information about the element, Cobalt, atomic number 27. Covers physical properties, atomic properties, how abundant it is on the Earth, and details about health-related regulations, and its importance to human and to animal health. Also...
Encyclopedia of Earth
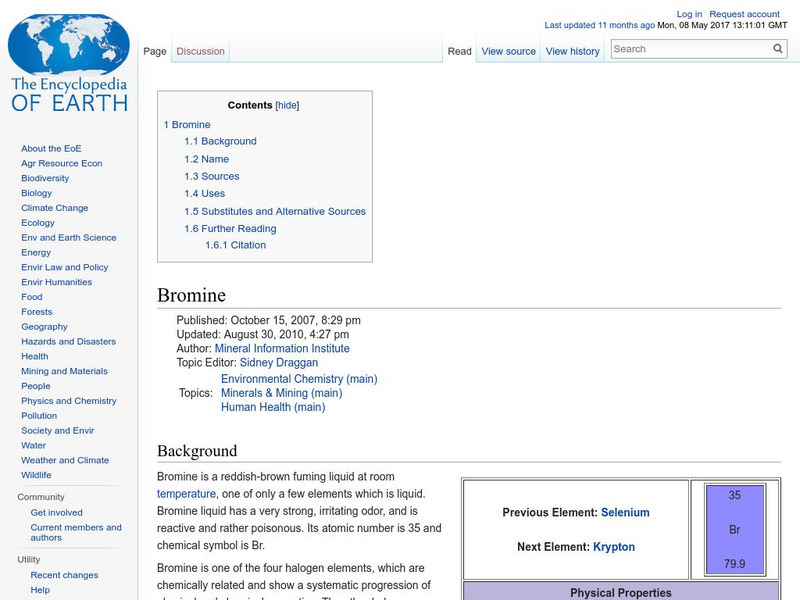
Encyclopedia of Earth: Bromine
Information about the element, Bromine, atomic number 35. Covers physical properties, atomic properties, how abundant it is on the Earth, and details about health-related regulations. Discusses sources, uses, and substitutes.
Other
Swiss Federal Institute of Technology: Pauli's Principle
Provides information concerning Wolfgang Pauli's Exclusion Principle. Contains the periodical table of elements.
National High Magnetic Field Laboratory
Magnet Academy: Enrico Fermi
Enrico Fermi was a titan of twentieth-century physics. He outlined the statistical laws that govern the behavior of particles that abide by the Pauli exclusion principle and developed a theoretical model of the atom in his mid-twenties....
Corrosion Source
Corrosion Source: Erbium
This site, which is provided for by Corrosion Source, gives a summary of basic physical data on erbium. This is a great site to check out for information on the subject.
Encyclopedia of Earth
Encyclopedia of Earth: Sodium
Information about the element, Sodium, atomic number 11. Covers physical and atomic properties, how abundant it is on the Earth, and human health considerations.