Sophia Learning
Sophia: Atomic Number
Learn what atomic number is, and what happens if the numbers of protons in an atom changes.
Purdue University
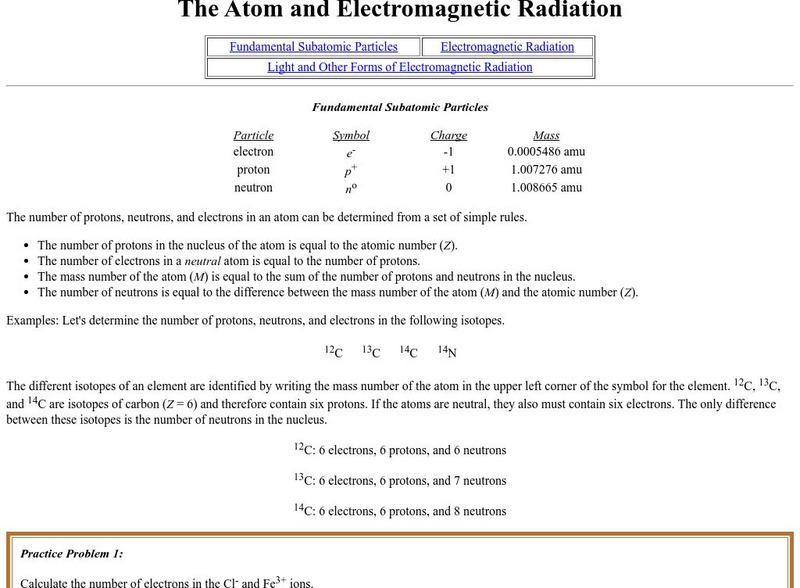
Purdue University: Fundamental Subatomic Particles
At this site from the Purdue University, the elementary subatomic particles are described and electromagnetic radiation is detailed. Includes learning exercises and answers.
BBC
Bbc: Gcse Bitesize: Atomic Structure
This lesson focuses on the structure of atoms. All substances are made from atoms. Each atom is made of a nucleus - containing protons and neutrons - surrounded by electrons. It provides a link to an assessment.
Sophia Learning
Sophia: Subatomic Particles: The Electron: Lesson 3
This lesson will explain that electrons are negatively charged particles with negligible mass and are found in pairs in orbitals surrounding the nucleus of an atom. It is 3 of 3 in the series titled "Subatomic Particles: The Electron."
Sophia Learning
Sophia: Subatomic Particles: The Neutron: Lesson 2
This lesson will explain that neutrons are particles in the nucleus that have no charge and a mass of one amu. It is 2 of 3 in the series titled "Subatomic Particles: The Neutron."
BBC
Bbc: Gcse Bitesize: What Does the Periodic Table Tell Us About the Elements?
The number of protons in the atom of an element determines its place in the Periodic Table. The number of electrons in an atom is the same as the number of protons. These electrons are arranged in shells or 'energy levels' around the...