CK-12 Foundation
Ck 12: Plix: Energy Levels: Bohr's Atomic Model
[Free Registration/Login Required] In this interactive you will excite an electron by dragging it into a higher orbital and use the table to see what wavelength of electromagnetic radiation is emitted when it returns to its lowest orbit....
Texas Education Agency
Texas Gateway: Atoms, Elements and the Periodic Table: The Atomic Model [Pdf]
A slideshow looking at the contributions of scientists over time to our understanding of atomic theory. Looks at models of the atom developed by Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick, as...
CK-12 Foundation
Ck 12: Atomic Theory
[Free Registration/Login may be required to access all resource tools.] In this online tutorial students will explain the law of conservation of mass, the law of definite proportions, and the law of multiple proportions. They will also...
Tom Richey
Slide Share: Atomic Models: Everything You Need to Know
A detailed slideshow for Grade 8 students that looks at the history of our understanding of atoms. Looks at the ideas presented by Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr, and the modern-day wave model. A...
CK-12 Foundation
Ck 12: Physics Simulation: Atomic Colors
[Free Registration/Login Required] Explore the Bohr model of atoms by studying the absorption and emission of light from simple gases in the context of starlight using this interactive simulation. A PDF worksheet and a video tutorial are...
CK-12 Foundation
Ck 12: The Bohr and Quantum Models of the Atom
[Free Registration/Login may be required to access all resource tools.] Students explore how the study of the hydrogen emission spectrum led to the Bohr model of the atom, in which electrons exist in states of constant energy.
PBS
Pbs: People & Discoveries Niels Bohr
A wonderful PBS biography of a great man. This contains many personal touches, yet does a fine job of describing Bohr's scientific work. But it's best at Bohr the man. Nice quotes, one in the middle, one at the end.
Other
Slide Player: History of the Atomic Model
Short slideshow looking at the history of models of the atom, including the contributions of Aristotle, Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr.
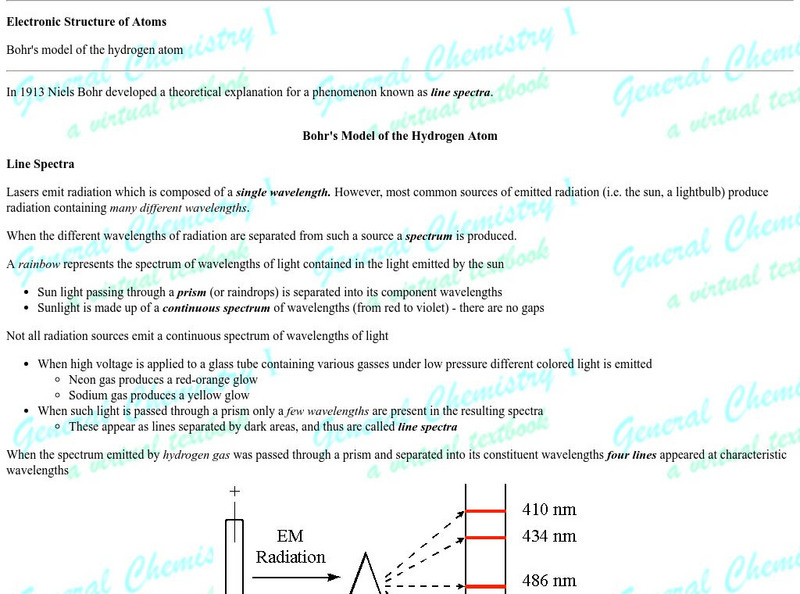
Simon Fraser University
Chem1 Virtual Textbook: Bohr's Theory
Acting as part of an overview on quantum theory, this section of the site answers the question, "How did Bohr's theory save the planetary model, for a while?" A section below also discusses the primary problems with Bohr's theory.
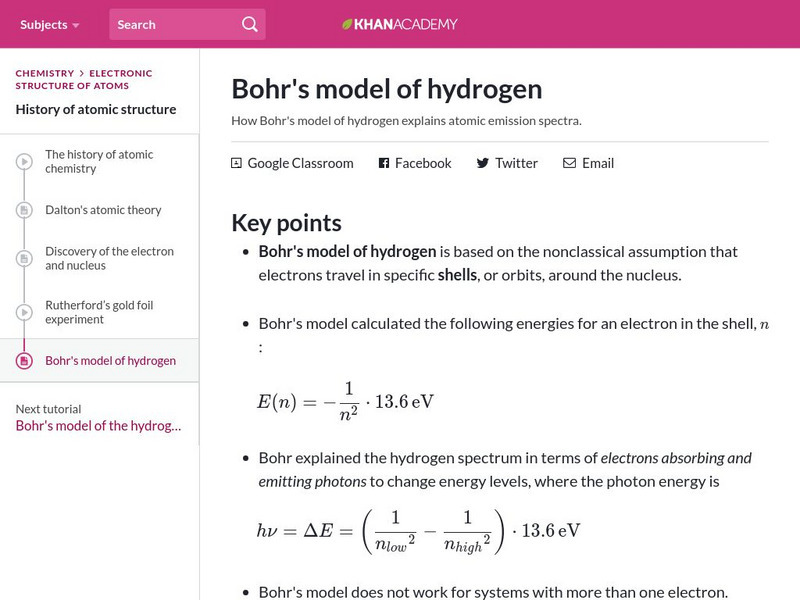
Khan Academy
Khan Academy: Bohr's Model of Hydrogen
Resource investigates how Bohr's model of hydrogen explains atomic emission spectra.
Nobel Media AB
The Nobel Prize: Niels Bohr Biographical
The Nobel Foundation provides this site about Niels Bohr's contributions to the world of physics, specifically his "investigation of the structure of atoms and of the radiation emanating from them." This biography includes information on...
Michael Blaber, PhD
Florida State University: The Bohr Model of the Atom
A well designed clear tutorial explaining the energies involved in the Bohr model of the atom. Illustrations add to the clearly presented equations.
Khan Academy
Khan Academy: Bohr's Model of Hydrogen
How Bohr's model of hydrogen explains atomic emission spectra.
Other
Iun: Modern Atomic Theory
This is an excellent site with information on the discovery of the atom and the different models. Includes a sample question and answer using Planck's constant.
Other
Cronodon: Atoms Models of the Atom
Presents models of the atom developed by Thomson, Rutherford, Bohr, and Schrodinger. Includes successes and failures of each, illustrations, and detailed descriptions. Also discusses Dirac's model of the atom and introduces quantum...
CK-12 Foundation
Ck 12: Modern Atomic Theory
[Free Registration/Login may be required to access all resource tools.] Rutherford's model of the atom was better than earlier models. But it wasn't the last word. Danish physicist Niels Bohr created a more accurate and useful model....
Famous Scientists
Famous Scientists: Aage Bohr
Learn about the life of Aage Niels Bohr, and see how his work was pivotal in the development of the theory of the structure of the atomic nucleus.
CK-12 Foundation
Ck 12: The Bohr Model of the Atom
[Free Registration/Login may be required to access all resource tools.] Students will learn about the history of atomic theory, and the development of the Bohr model of the atom. Includes a simulation for exploring the Bohr Model.
Tom Richey
Slide Share: Atomic Structure
Slideshow looking at the history of models of the atom, including those proposed by John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick. Discusses subatomic particles, including the numbers of protons, neutrons,...
CK-12 Foundation
Ck 12: Modern Atomic Theory
[Free Registration/Login may be required to access all resource tools.] Rutherford's model of the atom was better than earlier models. But it wasn't the last word. Danish physicist Niels Bohr created a more accurate and useful model....
Upper Canada District School Board
Tom Stretton's Advanced Placement Chemistry: Atomic Structure and Periodicity
This chemistry e-textbook provides students with AP-level reading and practice material on atomic structure and periodicity.
Boise State University
Boise State University: Atoms: A Virtual Field Trip Through Time and Space
Learn about the models of the atom that have been proposed throughout history. Presents Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and the modern theory of the atom. Sections are accompanied by journal...
Other
Chemtopics: Development of Modern Atomic Theory [Pdf]
A summary of the achievements of J. J. Thomson, Ernest Rutherford, Niels Bohr, and Erwin Schrodinger.
Simon Fraser University
Chem1 Virtual Textbook: Atoms and the Periodic Table
As part of the General Chemistry Virtual Textbook, this site examines a list of related topics (in hyperlink format) on the atom and the Periodic Table of elements. Topics from light, particles, and waves to the Bohr model, quantum...

![Texas Gateway: Atoms, Elements and the Periodic Table: The Atomic Model [Pdf] PPT Texas Gateway: Atoms, Elements and the Periodic Table: The Atomic Model [Pdf] PPT](https://d15y2dacu3jp90.cloudfront.net/images/attachment_defaults/resource/large/FPO-knovation.png)