Hi, what do you want to do?
Curated OER
Atoms, Molecules, and Chemical Bonds
In this atoms worksheet, students review the parts of an atom, Bohr diagram, atomic number, mass number, and covalent bonds. This worksheet has 5 drawings and 26 fill in the blank questions.
Curated OER
Review for Chemistry
In this review for chemistry worksheet, learners decide if given statements are true or false. Students relate information learned about introductory knowledge gained in chemistry to accurately answer the given questions.
Curated OER
Electrical Conduction in Semiconductors
In this electronics activity, students explore the properties of semiconductors to complete 17 short answer and problem solving questions.
Curated OER
If My Configurations are Correct
Students write the electron configuration of elements in the ground state. In this chemistry lesson, students draw how subatomic particles are arranged in the atom. They construct Lewis dot diagrams of valence electrons.
Curated OER
Physical and Chemical Changes
Eighth graders view a PowerPoint presentation that assist them in distinguishing between physical and chemical changes. They compare their observations of demonstrations to a list of clues recognizing types of changes.
Curated OER
Using several learning modalities to teach about the Periodic Table.
Students identify how to relate the position of an element in the periodic table to its atomic number and atomic mass. They identify how to use the periodic table to identify metals, semimetals, nonmetals, and halogens, and also,...
Curated OER
Static Cling
Students work together to discover the concept of static electricity. They participate in an experiment in which they test different objects charge. They make observations and record them for later use.
Curated OER
Quantum Physics
Students discuss the mass-energy relationship based on Einstein's work. They calculate the energy released in various scenerios and sketch diagrams for the Lyman, Balmer and Pfund Series. In groups, they discuss the role of photons and...
Curated OER
Typical Numeric Questions for Physics I - Atomic Spectra
Seven practice problems are presented to physics pros in this assignment. Given the wavelengths, they perform computations for emission spectra. This brief worksheet makes an appropriate pop quiz.
Khan Academy
Khan Academy: Bohr's Model of Hydrogen
Resource investigates how Bohr's model of hydrogen explains atomic emission spectra.
Michael Blaber, PhD
Florida State University: The Bohr Model of the Atom
A well designed clear tutorial explaining the energies involved in the Bohr model of the atom. Illustrations add to the clearly presented equations.
Khan Academy
Khan Academy: Bohr's Model of Hydrogen
How Bohr's model of hydrogen explains atomic emission spectra.
CK-12 Foundation
Ck 12: The Bohr Model of the Atom
[Free Registration/Login may be required to access all resource tools.] Students will learn about the history of atomic theory, and the development of the Bohr model of the atom. Includes a simulation for exploring the Bohr Model.
Other
Siyavula Education: Everything Maths & Science: Models of the Atom
Discusses models of the atom developed by John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick. Includes clear illustrations and a short comprehension exercise at the end.
Boise State University
Boise State University: Atoms: A Virtual Field Trip Through Time and Space
Learn about the models of the atom that have been proposed throughout history. Presents Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and the modern theory of the atom. Sections are accompanied by journal...
Other
Cronodon: Atoms Models of the Atom
Presents models of the atom developed by Thomson, Rutherford, Bohr, and Schrodinger. Includes successes and failures of each, illustrations, and detailed descriptions. Also discusses Dirac's model of the atom and introduces quantum...
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Models of the Hydrogen Atom
How did scientists figure out the structure of atoms without looking at them? Try out different models by shooting light at the atom. Check how the prediction of the model matches the experimental results.
Science Education Resource Center at Carleton College
Serc: Models of the Hydrogen Atom
In this activity, students will explore several different models of the hydrogen atom and compare and contrast them using an online java applet.
Other
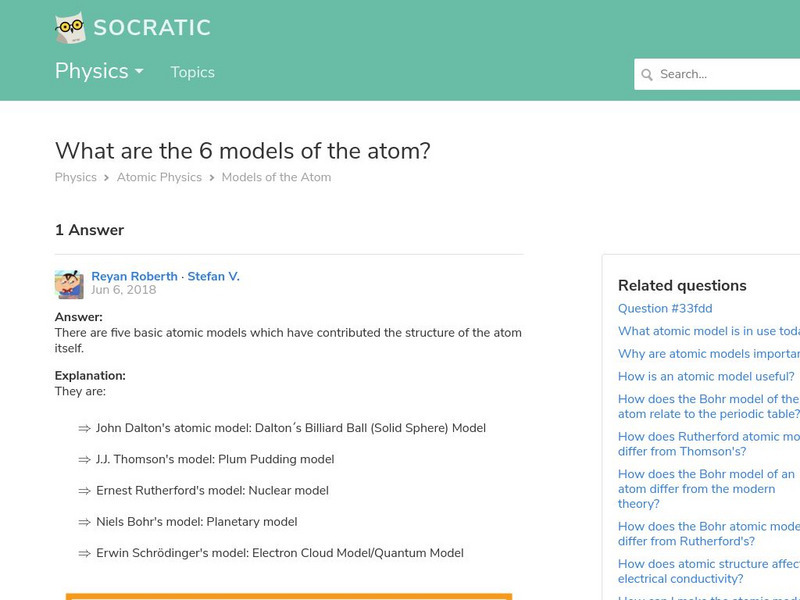
Socratic: What Are the 6 Models of the Atom?
Brief explanation of six major models of the atom along with illustrations. Covers the Greek model (Democritus), John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and the modern electron cloud or quantum mechanics model.
Texas Education Agency
Texas Gateway: Atoms, Elements and the Periodic Table: The Atomic Model [Pdf]
A slideshow looking at the contributions of scientists over time to our understanding of atomic theory. Looks at models of the atom developed by Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick, as...
Tom Richey
Slide Share: Atomic Models: Everything You Need to Know
A detailed slideshow for Grade 8 students that looks at the history of our understanding of atoms. Looks at the ideas presented by Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr, and the modern-day wave model. A...
Other
Crocodile Clips: Absorb Chemistry: History of the Atom
A tutorial that presents models of the atom proposed by John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr. Each is supported by an animated illustration. Includes comprehension questions and a quiz at the end.
CK-12 Foundation
Ck 12: The Bohr and Quantum Models of the Atom
[Free Registration/Login may be required to access all resource tools.] Students explore how the study of the hydrogen emission spectrum led to the Bohr model of the atom, in which electrons exist in states of constant energy.
Other
Erik's Chemistry Page: Electronic Structure of Atoms
A description of quantum theory, the Bohr model of the atom, the quantum mechanical atom, the Scrodinger equation, and quantum numbers.