Hi, what do you want to do?
Sophia Learning
Sophia: Models of the Atom: Lesson 3
Describe how the model of the atom advanced as scientific knowledge increased. This lesson is 3 of 3 in the series titled "Models of the Atom."
Science Struck
Science Struck: Ground State vs. Excited State of an Atom
Explains what Bohr's model of the atom is, the characteristics of the ground and excited states of an atom, and the electron configuration for each in the example of Phosphorus.
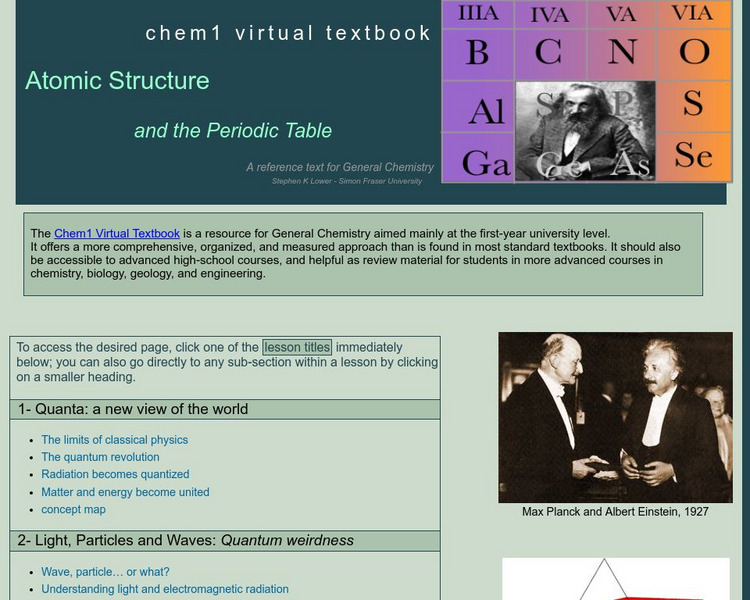
Simon Fraser University
Chem1 Virtual Textbook: Atoms and the Periodic Table
As part of the General Chemistry Virtual Textbook, this site examines a list of related topics (in hyperlink format) on the atom and the Periodic Table of elements. Topics from light, particles, and waves to the Bohr model, quantum...
Science Struck
Science Struck: The Structure of an Atom: A Labeled Diagram
Looks at the scientists who developed the model of the atom by building on previous scientists' discoveries about its structure.
Other
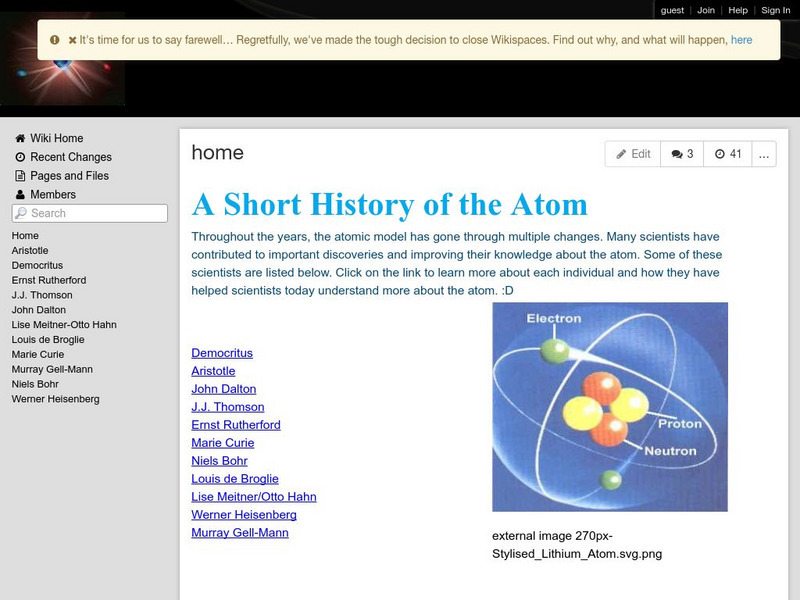
A Short History of the Atom
A classroom wiki where students present profiles of scientists who developed models of the atom or who contributed to the understanding of atomic theory. Covers Democritus, Aristotle, John Dalton, J.J. Thomson, Ernest Rutherford, Marie...
Tom Richey
Slide Share: Atomic Structure
Slideshow looking at the history of models of the atom, including those proposed by John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick. Discusses subatomic particles, including the numbers of protons, neutrons,...
Other
Slide Player: History of the Atomic Model
Short slideshow looking at the history of models of the atom, including the contributions of Aristotle, Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr.
Simon Fraser University
Chem1 Virtual Textbook: Bohr's Model
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses Niels Bohr and his work with the atom. This part of the discussion, focusing on the model itself, brings up...
CK-12 Foundation
Ck 12: Physics Simulation: Atomic Colors
[Free Registration/Login Required] Explore the Bohr model of atoms by studying the absorption and emission of light from simple gases in the context of starlight using this interactive simulation. A PDF worksheet and a video tutorial are...
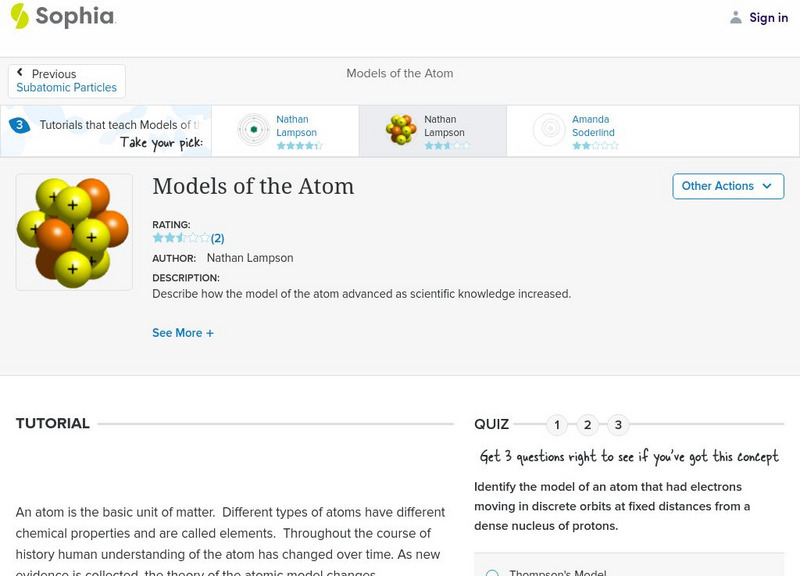
Sophia Learning
Sophia: Models of the Atom: Lesson 2
Describe how the model of the atom advanced as scientific knowledge increased. This lesson is 2 of 3 in the series titled "Models of the Atom."
Sophia Learning
Sophia: Models of the Atom: Lesson 1
Describe how the model of the atom advanced as scientific knowledge increased. This lesson is 1 of 3 in the series titled "Models of the Atom."
Walter Fendt
Walter Fendt: Bohr's Theory of the Hydrogen Atom
An explanation of Niels Bohr's (1885 - 1962 CE) Model along with an app that illustrates a hydrogen atom according to the particle or wave model. You can choose a principal quantum number "n." The right part of the graphic represents the...
Nobel Media AB
The Nobel Prize: Niels Bohr Biographical
The Nobel Foundation provides this site about Niels Bohr's contributions to the world of physics, specifically his "investigation of the structure of atoms and of the radiation emanating from them." This biography includes information on...
Other
Holonity: The Atom From Solid Balls to Sparkling Ghosts
Presents the history of the atom with a New Age perspective on some aspects. It describes the concept of the atom in Vedic philosophy and that developed by Democritus. Goes on to look at the models proposed by John Dalton, J.J. Thomson,...
University of Colorado
University of Colorado: Physics 2000: Bohr's Atom
An outstanding site that can best be described as student friendly. It describes the main differences between the classical and quantum models of the atom. Clear and well illustrated.
Simon Fraser University
Chem1 Virtual Textbook: Spectrum of the Hydrogen Atom
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses the hydrogen atom and its relation to spectrum. Included in the discussion is information on the Bohr model...
Other
Atoms in Motion: Atoms and Ions
All matter is made of atoms! And atoms are made of even smaller more fundamental particles. Atoms are not shiny little rigid spheres as we depict in drawings and simulations; they're more like a squishy storm of tiny particles swirling...
Simon Fraser University
Chem1 Virtual Textbook: The Bohr Atom
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses Niels Bohr and his work with the atom. Topics covered in the discussion include the atom before Bohr, Bohr's...
CK-12 Foundation
Ck 12: Modern Atomic Theory
[Free Registration/Login may be required to access all resource tools.] Rutherford's model of the atom was better than earlier models. But it wasn't the last word. Danish physicist Niels Bohr created a more accurate and useful model....
National High Magnetic Field Laboratory
Magnet Academy: Timeline of Electricity and Magnetism: 1910 1929
Scientists' understanding of the structure of the atom and of its component particles grows, the phone and radio become common, and the modern television is born.
CK-12 Foundation
Ck 12: Plix: Bohr's Atomic Model
[Free Registration/Login Required] Shown here are representations of three different models of the atom. Move the name labels to correctly title each of the models with the scientist who created them.
PBS
Pbs: Planck Discovers the Quantum Nature of Energy
PBS offers a short summary of the discovery of the quantum nature of the atom by Max Planck. Easy to follow.
CK-12 Foundation
Ck 12: Structure of the Atom
[Free Registration/Login may be required to access all resource tools.] Students learn about the important discoveries of subatomic particles, and how they led to our current understanding of the atom.
Khan Academy
Khan Academy: Spectroscopy: Interaction of Light and Matter
Tutorial provides a discussion of UV-Vis spectroscopy, infrared (IR) spectroscopy, and the Beer-Lambert law.