National Institute of Open Schooling
Atomic Structure
Learners explain historical findings such as Rutherford and Bohr's contributions, explain wave particle duality, and formulate Heinsenberg's uncertainty principle. They also draw s, p, and d orbitals, explain more historical findings,...
Michael Blaber, PhD
Florida State University: The Bohr Model of the Atom
A well designed clear tutorial explaining the energies involved in the Bohr model of the atom. Illustrations add to the clearly presented equations.
Other
Siyavula Education: Everything Maths & Science: Models of the Atom
Discusses models of the atom developed by John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick. Includes clear illustrations and a short comprehension exercise at the end.
Other
Cronodon: Atoms Models of the Atom
Presents models of the atom developed by Thomson, Rutherford, Bohr, and Schrodinger. Includes successes and failures of each, illustrations, and detailed descriptions. Also discusses Dirac's model of the atom and introduces quantum...
Simon Fraser University
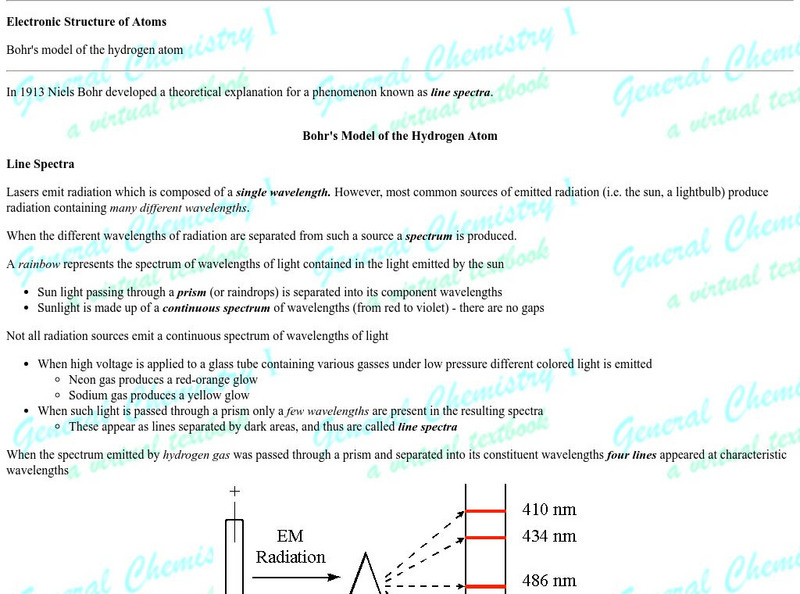
Chem1 Virtual Textbook: Spectrum of the Hydrogen Atom
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses the hydrogen atom and its relation to spectrum. Included in the discussion is information on the Bohr model...
Simon Fraser University
Chem1 Virtual Textbook: The Bohr Atom
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses Niels Bohr and his work with the atom. Topics covered in the discussion include the atom before Bohr, Bohr's...
Other
Erik's Chemistry Page: Electronic Structure of Atoms
A description of quantum theory, the Bohr model of the atom, the quantum mechanical atom, the Scrodinger equation, and quantum numbers.
Science Struck
Science Struck: Ground State vs. Excited State of an Atom
Explains what Bohr's model of the atom is, the characteristics of the ground and excited states of an atom, and the electron configuration for each in the example of Phosphorus.
Other
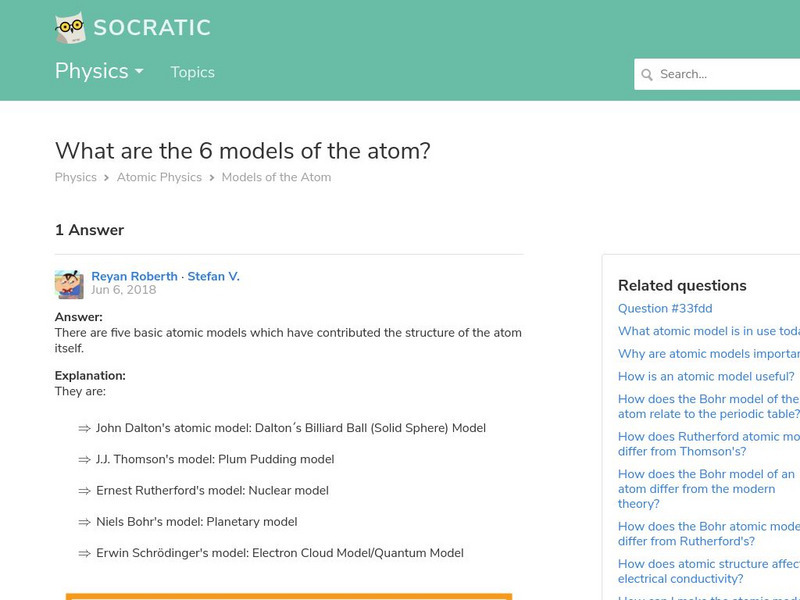
Socratic: What Are the 6 Models of the Atom?
Brief explanation of six major models of the atom along with illustrations. Covers the Greek model (Democritus), John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and the modern electron cloud or quantum mechanics model.
University of Colorado
University of Colorado: Physics 2000: Bohr's Atom
An outstanding site that can best be described as student friendly. It describes the main differences between the classical and quantum models of the atom. Clear and well illustrated.
Science Struck
Science Struck: The Structure of an Atom: A Labeled Diagram
Looks at the scientists who developed the model of the atom by building on previous scientists' discoveries about its structure.
Nobel Media AB
The Nobel Prize: Niels Bohr Biographical
The Nobel Foundation provides this site about Niels Bohr's contributions to the world of physics, specifically his "investigation of the structure of atoms and of the radiation emanating from them." This biography includes information on...
Friesian School
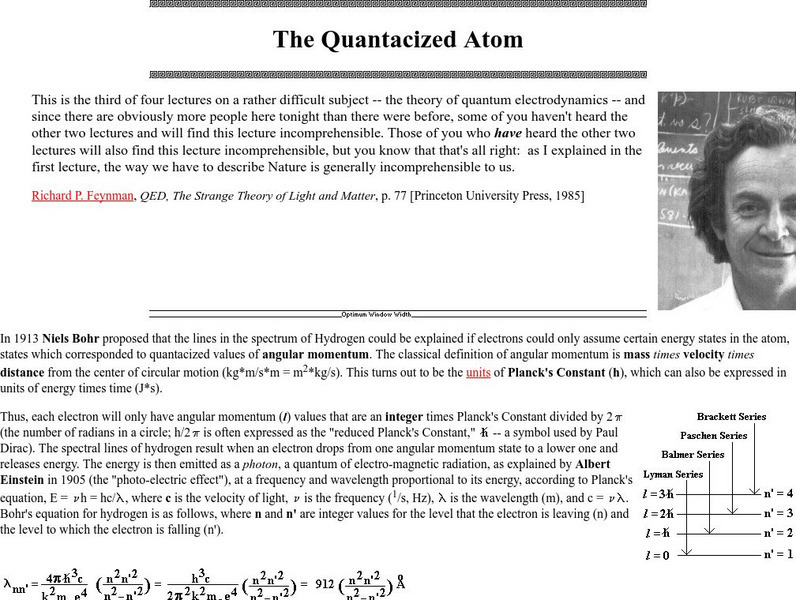
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Simon Fraser University
Chem1 Virtual Textbook: Spectrum of a Guitar String
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses spectrum in relation to Bohr's model. Included in the topics covered are standing waves, boundary condition,...
Lawrence Berkeley National Laboratory
Berkeley Lab: The Atom
Presented is an overview of atomic theory concentrating on the experiments of Ernest Rutherford.
Other
Iun: Modern Atomic Theory
This is an excellent site with information on the discovery of the atom and the different models. Includes a sample question and answer using Planck's constant.
National High Magnetic Field Laboratory
Magnet Academy: Timeline of Electricity and Magnetism: 1910 1929
Scientists' understanding of the structure of the atom and of its component particles grows, the phone and radio become common, and the modern television is born.
PBS
Pbs: Planck Discovers the Quantum Nature of Energy
PBS offers a short summary of the discovery of the quantum nature of the atom by Max Planck. Easy to follow.
Other
Fact index.com: Balmer Series
Fact-Index.com offers a brief dictionary definition of the Balmer series, including hyperlinked terms.
Other
Fact index.com: Paschen Series
Fact-Index.com offers a dictionary definition of the Paschen series, including hyperlinked terms.
Khan Academy
Khan Academy: Photoelectric Effect
Article explains the experiments on the photoelectric effect and how these experiments led to the idea of light behaving as a particle of energy called a photon.