National Institute of Open Schooling
p-Block Elements and Their Compounds – II
Ozone, made of three bonded oxygen atoms, is found 15-30 km above Earth, has a strong smell, is blue, and blocks sunlight from hitting the surface of Earth. The 22nd lesson in a series of 36 specifically focuses on the important elements...
National Institute of Open Schooling
Coordination Compounds
Cyanide, a coordination compound, is used in the extraction of gold and silver. Part 24 in the series of 36 delves into the world of coordination compounds. Classes learn, through readings, discussions, and answering questions, how to...
National Institute of Open Schooling
Chemical Bonding
Name is Bond, covalent bond. Through readings and answering questions, classes explore the different types of chemical bonds, their characteristics, valence shell electron pair repulsion theory, and atomic orbitals.
National Institute of Open Schooling
Alcohols, Phenols and Ethers
Classes continue their study of organic compounds in a detailed lesson covering alcohols, phenols, and ethers. Naming these compounds, classifying them, and describing their preparation and use are some of the topics covered. Through...
National Institute of Open Schooling
Nomenclature and General Principles
Carbon, the base for all organic compounds, exists in nature in its purest form as graphite or diamonds. The 25th lesson in a series of 36 teaches pupils the nomenclature of organic compounds. Learners read about how to use the IUPAC...
National Institute of Open Schooling
Aldehydes, Ketones and Carboxylic Acids
Although their name makes them sound dangerous or toxic, carboxylic acids are found throughout nature in things such as citric acid, vinegar, and even in your DNA. Through detailed readings, discussions, and answering questions...
National Institute of Open Schooling
General Characteristics of the p-Block Elements
The 20th installment in a series of 36 focuses on the characteristics of the p-block elements. Learners discuss, read about, and answer questions pertaining to the occurrence of these elements in nature, their electron configurations,...
Michael Blaber, PhD
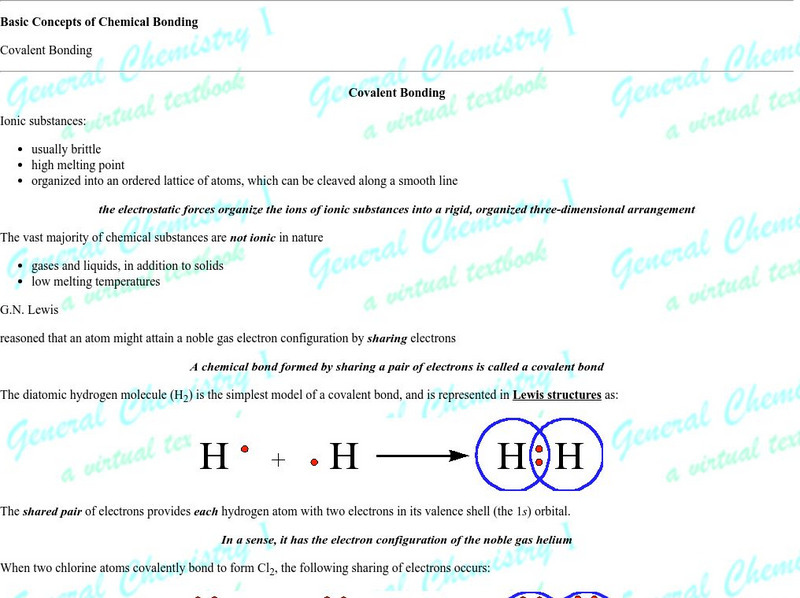
Florida State University: Basic Concepts of Covalent Bonding: Covalent Bonding
Good introduction and graphics make this a solid page for understanding the orbital role in bonding and molecular geometry. The author is a professor at Florida State University.
Chem Tutor
Chem Tutor: Binary Covalent Compounds
An explanation of bonding in binary covalent compounds. Rules for naming binary covalent compounds using common names and system names are also provided.
National Institutes of Health
Ncbi: The Molecular Biology of the Cell: The Chemical Components of a Cell
Advanced chapter of the book "The Molecular Biology of the Cell" describes and provides illustrations of our most current understanding of the chemical makeup of cells and their components. Explains in detail how electron activity keeps...
Wikimedia
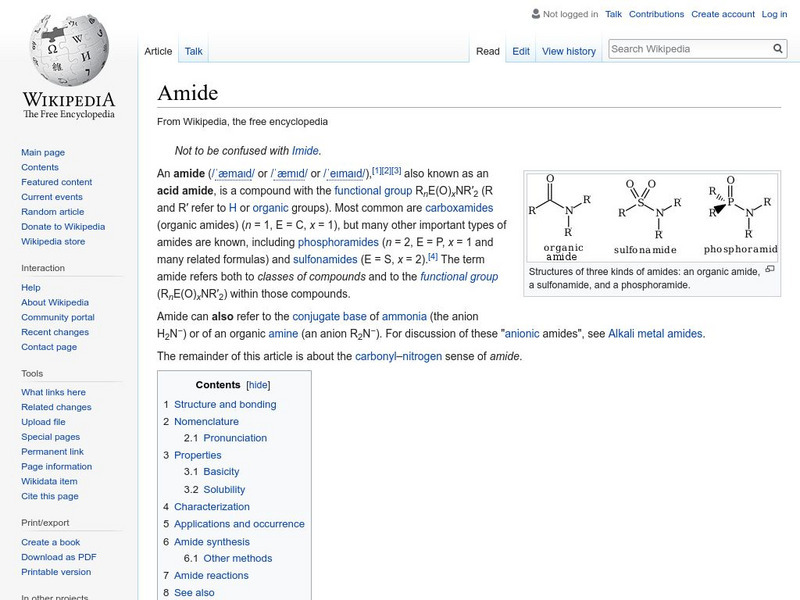
Wikipedia: Amide
Wikipedia entry for the chemical compound amide. Includes naming conventions, properties, reactions, and so on.
Wikimedia
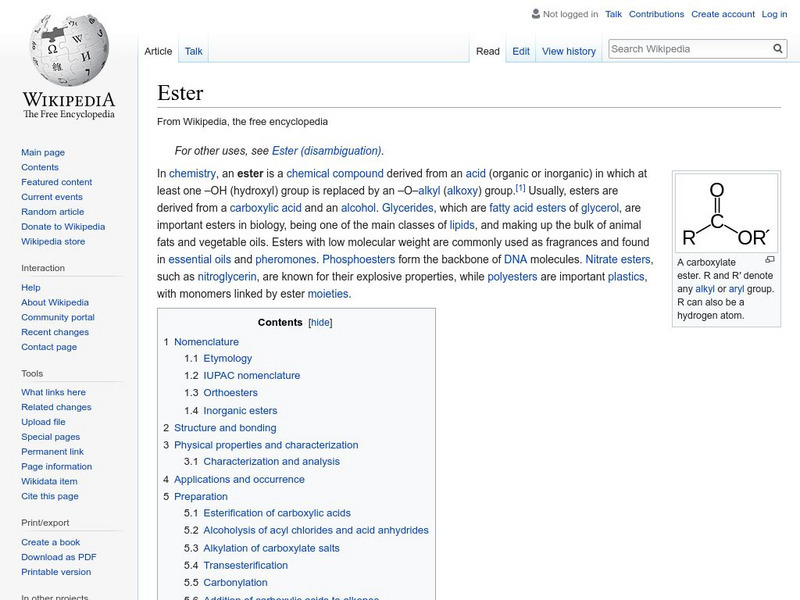
Wikipedia: Ester
Wikipedia entry for the chemical compound ester. Includes naming conventions, properties, reactions, and the like.
Towson University
Towson University: Drawing Lewis Structures
Brief rules for writing Lewis structures for simple compounds. Some examples are included.