Open Curriculum
Open Curriculum: Molar Mass
The objective of this article is to compute molar masses of chemicals and to use molar masses in conversion problems.
CK-12 Foundation
Ck 12: Chemistry Simulation: Mole Carnival
[Free Registration/Login Required] In the mole carnival, students count the number of atoms and particles in different objects found at the carnival. As students work to find the number of particles, they practice counting particles by...
Crescent Public Schools
The Internet Science Room: P H and the P H Scale
A chemistry tutorial which explains pH and the pH scale. There are also opportunities for students to do some calculation practice.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Molar Mass Conversion Factors
Read how to use molar mass equations to find conversion factors and molecular compounds. View several examples using given formulas for molar mass.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Molar Mass and Chemical Compounds: Audio Book
Listen and watch as this audio book shows the relations of molar mass and chemical compounds. Discover the formula for molecular mass, ionic compounds, and formula units, and view several pictures about chemical formulas.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Molar Mass Conversion Factors
Test your knowledge of moles by practicing this animated exercise. Fill in the conversion factors for the molar mass and see if you are a mole master!
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Molarity and Equation Stoichiometry: Audio Book
This audio book, narrated by author Mark Bishop, describes how to use equation stoichiometry for reactions that are in a solution. Many examples are shown, and general steps to complete the problems are given.
Georgia Department of Education
Ga Virtual Learning: Ap Chemistry: Stoichiometry
Students apply new knowledge of the elements to name and write chemical formulas, write and balance chemical equations, and perform mathematical calculations about the relationships between those chemicals, using a fundamental unit in...
Crescent Public Schools
The Internet Science Room: The Mole Concept
Students explore the concept of a mole and molar mass, and how these values are used in chemistry.
Frostburg State University
General Chemistry: Gaseous Equation of State Calculator
A JavaScript calculator which allows one to investigate the distinction between an ideal gas and a real (Van der Waal or Dieterici) gas. State variables can be entered and an update on the values of other variables are given (as...
Frostburg State University
General Chemistry Online: The Mole Concept
Resource provides notes on Moles and Stoichiometry. Deals with all book-keeping aspects, including as section on yields and limiting reactants. Includes lesson plans, lecture slides and notes, links to related websites, and frequently...
Oswego City School District
Regents Exam Prep Center: Moles & Stoichiometry: Moles Resource
A nice page of links to follow for learning about moles. Contains links to sites that have explanations of moles and molar mass, practice problems, and more.
TED Talks
Ted: Ted Ed: How Big Is a Mole? (Not the Animal, the Other One.)
Video describes how moles are used in chemistry and how their size is measured. [4:33] Followed by a short quiz and a list of additional resources to explore.
Cosmo Learning
Cosmo Learning: General Chemistry
A collection of video lectures from a general chemistry course taught at the University of California, Berkeley. The course teaches periodic table, chemical bonds, molecular shape, phase changes, chemical reactions, stoichiometry,...
Science Education Resource Center at Carleton College
Serc: Molar Relationships: Observing a Single Displacement Reaction
In this chemistry lab, students will investigate a single replacement reaction while also using knowledge of molar ratios to determine percent yields.
Upper Canada District School Board
Tom Stretton's Advanced Placement Chemistry: Thermochemistry
This chemistry e-textbook provides students with AP-level reading and practice material on thermochemistry.
Upper Canada District School Board
Tom Stretton's Advanced Placement Chemistry: Stoichiometry
This online textbook chapter provides learners with advanced-level reading and practice material on stoichiometry
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Chemical Calculations and Equations: Study Guide [Pdf]
Use this study guide to help review information about chemical calculations and chemical equations. Study everything from equation stoichiometry to molarity of substances.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Chemical Calculations and Chemical Equations [Pdf]
This chapter on chemical calculations and equations focuses on equation stoichiometry. Learn how to convert masses, use limiting reactants, and calculate the molarity of a solution.
California State University
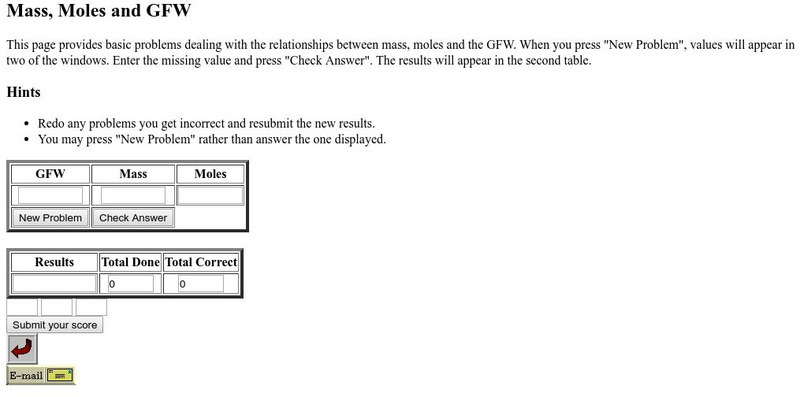
Csudh Project for Chemistry: Mass, Moles, and Gfw
Several basic problems dealing with the relationships between mass, moles, and the GFW.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Percent Element in Compound
This section from the online textbook, "An Introduction to Chemistry", gives the steps to calculate the percent of an element in a compound. A few example calculations are shown. Also find links to chapter maps and power points for other...
University of Florida
Chemistry 2041 Lecture Notes: Ideal Gases
The ideal gas law is presented and explained. The derivation of other gas laws is performed. Gas behavior is explained in terms of gas laws. Excellent graphics.
Chemistry Collective
Chem Collective: Glucose Dilution Problem
In this activity, students use the virtual lab to create a 0.025M glucose solution from a standard 1M glucose solution. First, they calculate the correct volumes of 1M glucose solution and water to mix together to create the final 0.025M...
Chemistry Collective
Chem Collective: Determination of the P H Scale
This virtual lab activity where students perform the method of successive dilutions using HCl, NaOH, a pH meter, and universal indicator solution to help understand the logarithmic nature of the pH scale.