CK-12 Foundation
Ck 12: Aqueous Solutions
[Free Registration/Login may be required to access all resource tools.] In this learning module, students will study water's ability to act as a solvent.
Khan Academy
Khan Academy: Water Autoionization and Kw
Learn about the autoionization of water, the autoionization constant Kw, and the relationship between [H+] and [OH-] in aqueous solutions.
Simon Fraser University
Chem1 Virtual Textbook: Electrolytic Cells and Electrolysis
As part of the General Chemistry Virtual Textbook, this site examines a variety of topics related to electrochemistry. In a discussion of electrolysis, this site covers topics including electrolysis in aqueous solutions, Faraday's laws...
Chemistry Collective
Chem Collective: Determining the Heat of Reaction in Aqueous Solution
In this activity, students perform an experiment to determine the heat of a reaction.
CK-12 Foundation
Ck 12: Chemistry: Solute and Solvent
[Free Registration/Login may be required to access all resource tools.] Covers solutes and solvents.
CK-12 Foundation
Ck 12: Electrolysis
[Free Registration/Login may be required to access all resource tools.] Students investigate voltaic and electrolytic cells describing the reactions that occur during the electrolysis of water. They also identify the products that would...
Science Education Resource Center at Carleton College
Serc: Do Water Molecules Have Space Between Them?
In this chemistry lab, students investigate whether water molecules have any space between them by filling a glass with water, and adding salt without the water overflowing. They will also experiment with the temperature of the water.
CK-12 Foundation
Ck 12: Solubility Equilibrium
[Free Registration/Login may be required to access all resource tools.] Students write the solubility product constant expressions for nearly insoluble ionic compounds and perform calculations for compounds and solubility.
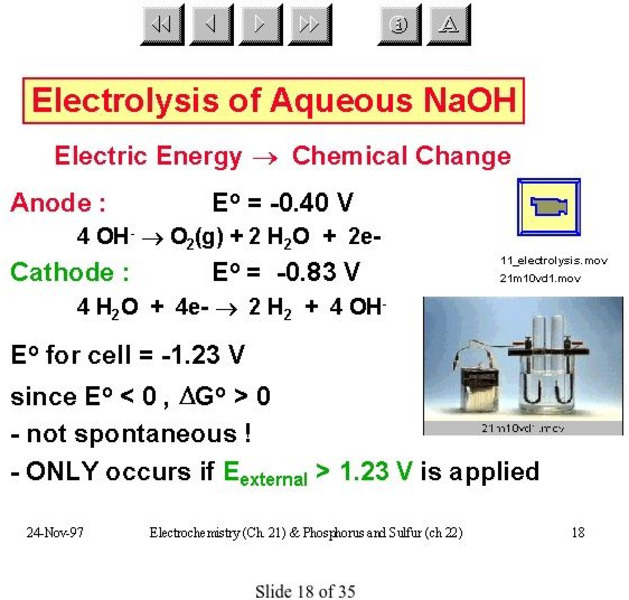
McMaster University
Mc Master University: Electrolysis of Aqueous Na Oh
Experimental setup and energy requirements for electrolysis of sodium hydroxide solution.