Texas Instruments
Texas Instruments: Half Life Lab
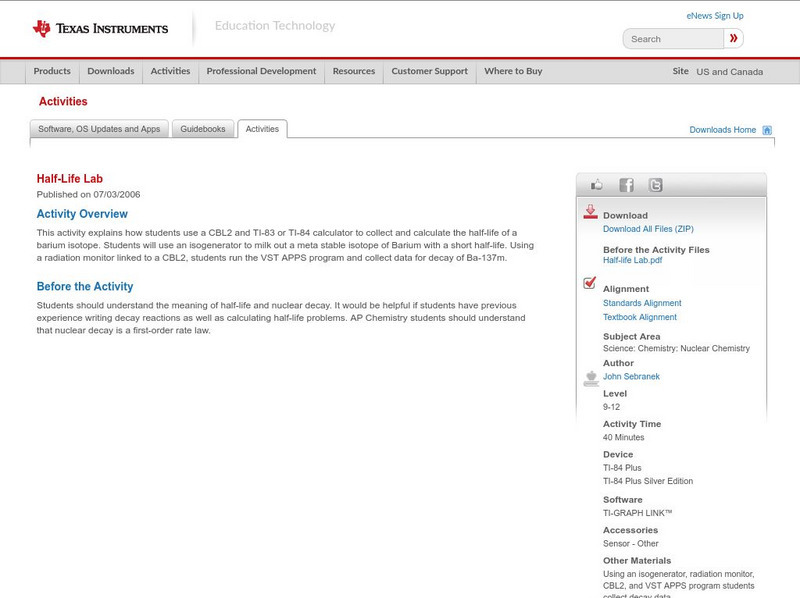
This activity explains how students use a CBL2 and TI-83 or TI-84 calculator to collect and calculate the half-life of a barium isotope. Students can use an isogenerator to milk out a meta stable isotope of Barium with a short half-life....
Other
New Mexico State University: Change in Entropy
An example of a calculation of the change in entropy in a simple chemical reaction.
Khan Academy
Khan Academy: Bond Enthalpy and Enthalpy of Reaction
Explanation of bond enthalpy that includes examples of calculating enthalpies of reaction using bond enthalpy.
CK-12 Foundation
Ck 12: Percent Composition and Empirical & Molecular Formulas
[Free Registration/Login may be required to access all resource tools.] In this online tutorial students will learn to calculate the percent composition of a compound either from mass data or from the chemical formula. They will use...
CK-12 Foundation
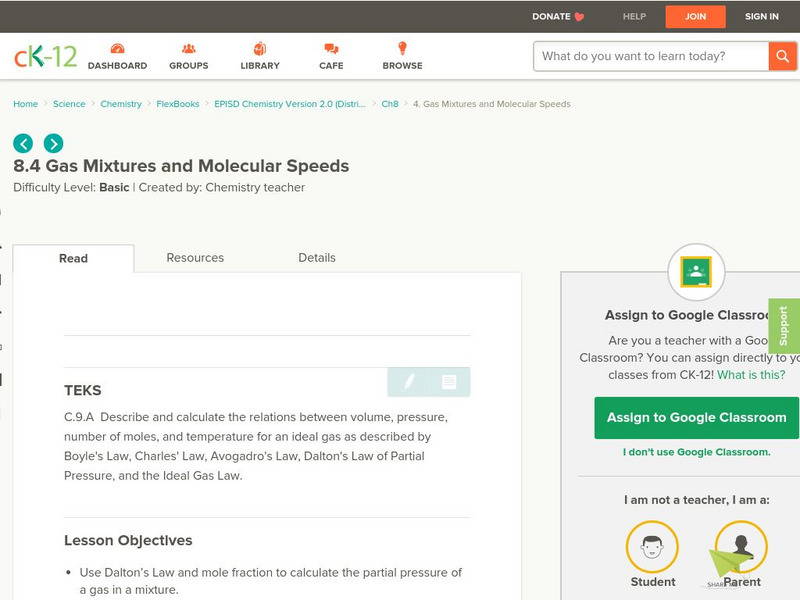
Ck 12: Gas Mixtures and Molecular Speeds
[Free Registration/Login may be required to access all resource tools.] The following online tutorial helps students use Dalton's Law and mole fraction to calculate the partial pressure of a gas in a mixture. They will learn to calculate...
Khan Academy
Khan Academy: Heat and Temperature
An explanation of using heat capacity to calculate heat.
Khan Academy
Khan Academy: Pressure Volume Work
Work, in regards to thermodynamics, is defined and calculated using the expansion and contraction of gasses.
CK-12 Foundation
Ck 12: Limiting Reactant and Percent Yield
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students wil begin to analyze a chemical reaction in order to determine which reactant is the limiting reactant and which is the...
CK-12 Foundation
Ck 12: Ideal Gases and Gas Stoichiometry
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will learn the Ideal Gas Law, and know which of the different values for the ideal gas constant to use in a given...
CK-12 Foundation
Ck 12: Solution Concentration
[Free Registration/Login may be required to access all resource tools.] Students will calculate the concentration of solutions in units of molarity, and use molarity to calculate the dilutions of solutions.
CK-12 Foundation
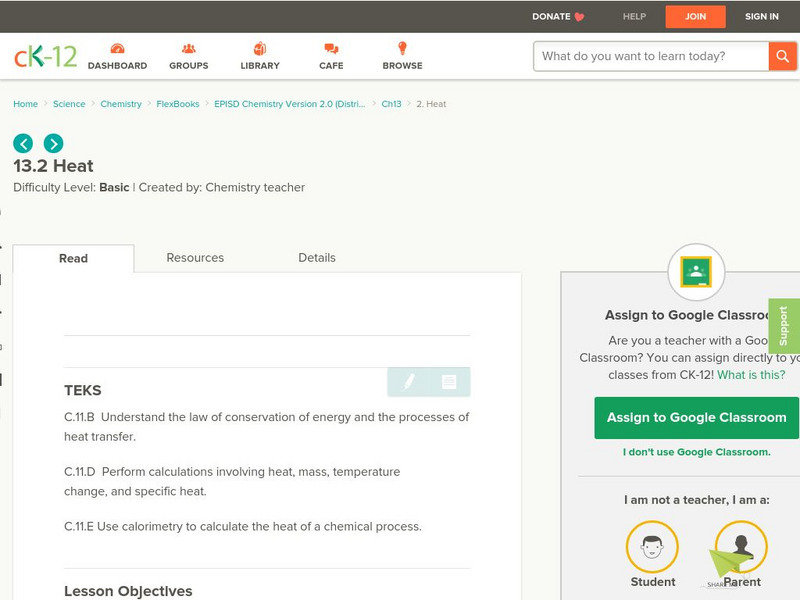
Ck 12: Heat
[Free Registration/Login may be required to access all resource tools.] Using the law of conservation of energy as a starting off point, students use the specific heat equation to perform calculations that relate mass, specific heat,...
CK-12 Foundation
Ck 12: Chemical Formulas
[Free Registration/Login may be required to access all resource tools.] Students will use a chemical formula or mass data to calculate the percent composition of a compound, and then calculate the empirical or molecular formula for a...
CK-12 Foundation
Ck 12: Uncertainty in Measurements
[Free Registration/Login may be required to access all resource tools.] In the following online lesson students will learn to distinguish between accuracy and precision in measurements and to also calculate the percent error of a...
CK-12 Foundation
Ck 12: Unit Conversions
[Free Registration/Login may be required to access all resource tools.] In this online tutorial students will begin to identify and use conversion factors and to also use the method of dimensional analysis to convert between units....
CK-12 Foundation
Ck 12: Isotopes and Atomic Mass
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will be asked to define atomic number, mass number and understand how isotopes differ from one another. They will be able...
CK-12 Foundation
Ck 12: The Quantum Mechanical Model
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will calculate the wavelength, frequency, and energy of light using Planck's constant and the speed of light. They will...
CK-12 Foundation
Ck 12: The Mole Concept
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will identify three methods for measuring the amount of matter in a sample. They will define the mole and its relationship...
CK-12 Foundation
Ck 12: Solubility Equilibrium
[Free Registration/Login may be required to access all resource tools.] Students write the solubility product constant expressions for nearly insoluble ionic compounds and perform calculations for compounds and solubility.
CK-12 Foundation
Ck 12: The P H Concept
[Free Registration/Login may be required to access all resource tools.] In this module, students will define pH and use the hydrogen or hydroxide ion concentrations to calculate the pH of a solution. They will also differentiate among...
CK-12 Foundation
Ck 12: Acid and Base Strength
[Free Registration/Login may be required to access all resource tools.] Help distinguish between degrees of dissociation for strong and weak acids and bases, and then define pH and use the hydrogen or hydroxide ion concentrations to...
CK-12 Foundation
Ck 12: Salt Solutions
[Free Registration/Login may be required to access all resource tools.] Students begin by predicting whether a salt solution is acidic, basic, or neutral, and then practice writing balanced equations for hydrolysis reactions. They also...
CK-12 Foundation
Ck 12: Nuclear Radiation
[Free Registration/Login may be required to access all resource tools.] In this module, students will understand how radioactivity involves a change in the nucleus of a radioisotope and calculate the conversion of mass to energy...
CK-12 Foundation
Ck 12: Spontaneous Reactions and Free Energy
[Free Registration/Login may be required to access all resource tools.] Students discover the concept of a spontaneous reaction in terms of enthalpy and entropy changes, and then investigate and calculate free energy.
CK-12 Foundation
Ck 12: Mole Ratios
[Free Registration/Login may be required to access all resource tools.] In this learning module, students will learn how to calculate and account for the amounts of reactants and products in a given chemical reaction.