Hi, what do you want to do?
Chemistry Collective
Chem Collective: Stoichiometry Tutorials: Dimensional Analysis
Watch a short tutorial showing the conversion between the amount of a substance expressed in number of molecules to the amount of a substance expressed in moles of molecules, and then try some dimensional analysis practice problems.
CK-12 Foundation
Ck 12: Stoichiometric Calculations
[Free Registration/Login may be required to access all resource tools.] Based on the balanced chemical equation, students will calculate the masses or moles of reactants or products generated in a given reaction. They will also convert...
Simon Fraser University
Chem1 Virtual Textbook: Molar Mass of a Gas Mixture
The General Chemistry Virtual Textbook, or Chem 1, is broken into several sections covering various aspects of topics related to chemistry. This section deals with the molar mass of gas mixtures. Applications of Dalton's law are also...
Other
Widener University: Ideal Gas Law
At this site from the Widener University Department of Chemistry, you can try your skill with using the ideal gas law equation to solve problems. This page contains an interactive JavaScript form which delivers problem after problem. You...
Wyzant
Wyzant: Chem Tutor: Gases
A lengthy page covering most all the gas laws. Each law is described in words and stated as an equation. The use of each law in solving problems is demonstrated. Some problem-solving tips are provided. There are 23 practice problems with...
University of Maryland
University of Maryland: Ideal Gas Law Volume of One Mole
A page from the University of Maryland Physics Lecture Demonstration Facility. Provides directions for a teacher demonstration of the ideal gas law. Shows apparatus and set-up; provides suggestions. Easily adaptable as a student project...
Iowa State University
Iowa State University: Argument for Scientific Realism
This page from Iowa State University describes the various experimental ways that Avogadro's number has been determined.
Other
Lock Haven: Glossary of Frequently Misused or Misunderstood Physics Terms
A page, written by Donald E. Simanek of Lock Haven University discusses misused and misunderstood physics terms and concepts. It reminds us that Avogadro's number is a constant, and should be treated as such.
CK-12 Foundation
Ck 12: Stoichiometric Calculations
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will learn to calculate the amount in moles of a reactant or product from the mass of another reactant or product. They...
CK-12 Foundation
Ck 12: Limiting Reactant and Percent Yield
[Free Registration/Login may be required to access all resource tools.] Students will compare theoretical yield to actual yield, and then investigate what happens when one reactant runs out before the other reactants are fully consumed....
CK-12 Foundation
Ck 12: Gas Properties
[Free Registration/Login may be required to access all resource tools.] The following online tutorial describes how a gas can be compressed and identifies three factors that affect gas pressure. Students will describe the effects...
CK-12 Foundation
Ck 12: Solubility Equilibrium
[Free Registration/Login may be required to access all resource tools.] Students write the solubility product constant expressions for nearly insoluble ionic compounds and perform calculations for compounds and solubility.
Khan Academy
Khan Academy: Stoichiometry
How to use mole ratios from a balanced reaction to calculate amounts of reactants.
Indiana University
Indiana University Northwest: Molar Mass
This site shows how to compute molar mass by working through an example.
Crescent Public Schools
The Internet Science Room: Solution Concentration
Students have the opportunity to study examples and worked out example problems to further their understanding of chemical solution concentration.
Chemistry Collective
Chem Collective: Glucose Dilution Problem
In this activity, students use the virtual lab to create a 0.025M glucose solution from a standard 1M glucose solution. First, they calculate the correct volumes of 1M glucose solution and water to mix together to create the final 0.025M...
Chemistry Collective
Chem Collective: Acid Dilution Problem
In this activity, students use the virtual lab to create 500mL of 3M HCl solution from a concentrated stock solution of 11.6M HCl. They must first calculate the correct volumes of 11.6M HCl solution and water to mix together to create...
Chemistry Collective
Chem Collective: Making Stock Solutions From Solids
In this activity, students use the virtual lab to create stock solutions starting from solid salts. Students must first calculate the correct amount of solid to make the solution. Next, they prepare the solution using the appropriate...
Other
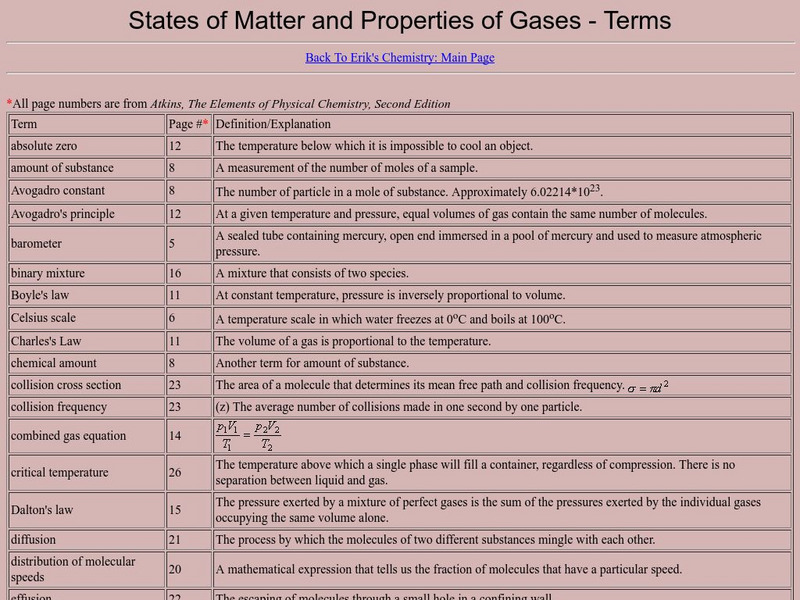
States of Matter and Properties of Gases: Terms
A very complete list of terms that are important to the study of gases. This resource is a web archive.