Davidson College
Davidson College: Valence Shell Electron Pair Repulsion (Vsepr) Model
Presents exercises where students can check their understanding of the Valence-Shell Electron-Pair Repulsion Model by predicting what a molecular shape will be. Requires Java.
CK-12 Foundation
Ck 12: Molecular Geometry
[Free Registration/Login may be required to access all resource tools.] The following online tutorial explains the basis of VSEPR theory. It helps students predict the shapes of molecules and polyatomic ions using VSEPR theory and it...
Upper Canada District School Board
Tom Stretton's Advanced Placement Chemistry: Chemical Bonding
Take on this self-guided advanced level e-text, and learn about chemical bonding and molecular structure.
Simon Fraser University
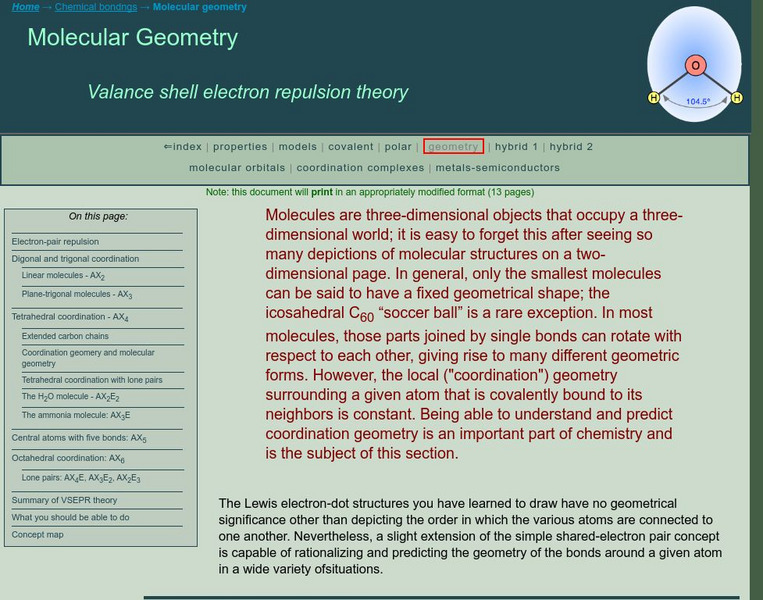
Chem1 Virtual Textbook: Molecular Geometry
An advanced explanation of the valence shell electron pair repulsion (VSEPR) theory describes specific molecular models involving digonal, trigonal, tetrahedral, and octahedral coordination, as well as central atoms with five bonds....
Towson University
Towson University: Shapes of Molecules
This chemistry class printout details the main points of molecular geometry and explains bond hybridization and bond angles.
Chem Tutor
Chem Tutor: Chemistry: Compounds
This instructional activity focuses on chemical compounds including Ionic and Covalent Bonds, Valences, Lewis Structures, Binary Covalent Compounds, Radicals or Polyatomic Ions, and much more. It also includes a compound worksheet in...
CK-12 Foundation
Ck 12: Molecular Geometry
[Free Registration/Login may be required to access all resource tools.] In this interactive learning module, students will learn a technique to predict molecular geometry based on a molecule's Lewis electron dot structure.
Wolfram Research
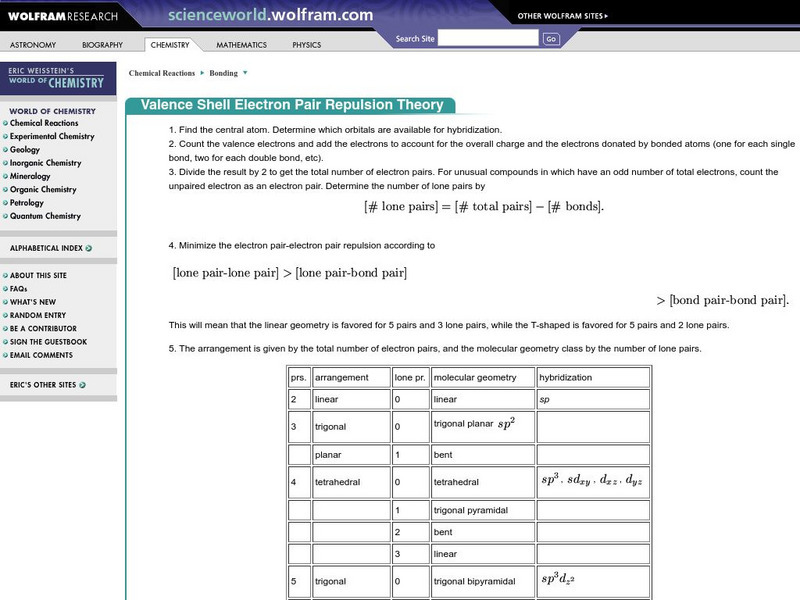
Wolfram Science World: Valence Shell Electron Pair Repulsion
Good site includes the basics of arrangement, hybridization, and gemetry.
McMaster University
Mc Master University: Molecular Structure
This PowerPoint presentation features 33 slides that explain chemical bonding and molecular shape.