Hi, what do you want to do?
Science Education Resource Center at Carleton College
Serc: Lab: Conservation of Mass
This lab experiment shows students that mass is not gained or lost during a chemical reaction. Lab also includes a set of lab questions that requires students to balance the chemical equation for the reaction that occurs in this lab.
Khan Academy
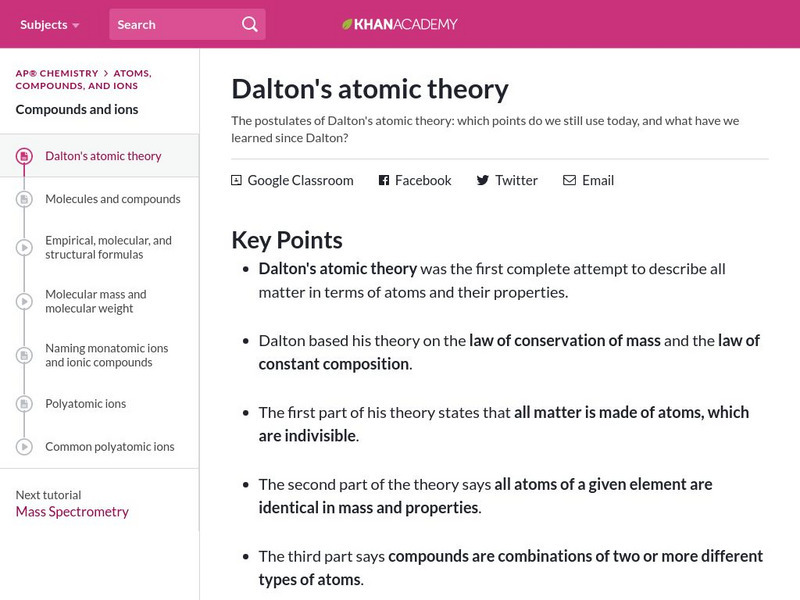
Khan Academy: Dalton's Atomic Theory
Article explores the key points of Dalton's atomic theory and the laws of conservation of mass and constant composition. Which points do we still use today?
Open Curriculum
Open Curriculum: Conservation of Energy
This Kahn Academy tutorial helps students understand the principles of conservation of energy and mass. [10 min, 6 sec]
BBC
Bbc: Gcse Bitesize: Calculations for All Students
Law of conservation of mass: No atoms are created or destroyed in a chemical reaction. They just join together in a different way than they were before the reaction, and form products. This means that the total mass of the products in a...
CPALMS
Florida State University Cpalms: Florida Students: Conservation of Mass Tutorial
Learn how mass is conserved in physical and chemical changes.
Utah Education Network
Uen: Physical Changes
Second graders explore the concept of law of conservation of mass.
CK-12 Foundation
Ck 12: Atomic Theory
[Free Registration/Login may be required to access all resource tools.] In this online tutorial students will explain the law of conservation of mass, the law of definite proportions, and the law of multiple proportions. They will also...
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Masses & Springs
An interactive simulation that teaches about springs, Hooke's Law, and conservation of energy observing changes in kinetic, potential, and thermal energy by adjusting the stiffness and damping of springs holding various masses. This...
The Wonder of Science
The Wonder of Science: Ms Ps1 5: Conservation of Atoms in Reactions
Work samples, phenomena, assessment templates, and videos that directly address standard MS-PS1-5: conservation of atoms in reactions.
The Wonder of Science
The Wonder of Science: 5 Ps1 2: Conservation of Matter
On this site, find a variety of materials to help students understand the conservation of matter. Create lessons that allow students to measure and graph quantities that could show even if matter is heated, cooled or combined with other...
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Pendulum Lab
An interactive simulation that teaches about periodic motion, simple harmonic motion, and conservation of energy. Observe one or two pendulums to determine how the swing is influenced by the length of the string, the mass of the pendulum...
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Masses and Springs
Use this interactive, animated lab to explore conservation of mechanical energy.
Better Lesson
Better Lesson: Changing Matter:changing Matter: Is Weight the Same or Different?
Learners conduct a hands-on investigation to determine how matter, changing state, effects the property of weight. Students will collect data and graph their results. Resources include step by step instructions, a data worksheet, videos...
CK-12 Foundation
Ck 12: Chemical Equations
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will describe chemical reactions using word equations and know the correct symbols to use in order to write skeleton...
Texas Education Agency
Texas Gateway: Matter and Energy: Chemical Equations
Explore balanced chemical equations in this tutorial. Tutorial includes videos, interactive activities, and practice to help students understand balanced chemical equations and the Law of Conservation of Mass.
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Masses & Springs
Hang various mass weights on spring scales while you adjust the spring stiffness and damping in this online activity. Slow down the action, take it to another planet, or watch the amount of potential, thermal, and kinetic energy.
Khan Academy
Khan Academy: Dalton's Atomic Theory
An article describing Dalton's atomic theory. Learn how this theory was the first attempt to describe a fundamental part of matter, atoms. Article also discusses where the theory is incorrect based on the knowledge we have today.
CK-12 Foundation
Ck 12: Fifth Grade: Physical Science: Physical and Chemical Changes in Matter
[Free Registration/Login may be required to access all resource tools.] Provides the definitions of physical and chemical changes in matter and gives examples. The law of conservation of mass is also described.
Khan Academy
Khan Academy: Dalton's Atomic Theory
Resource investigates the beliefs of Dalton's atomic theory which consists of four parts. Which points do we still use today, and what have we learned since Dalton?
Georgia Department of Education
Ga Virtual Learning: Ap Physics 1: Rotational Dynamics
Students learn about the Newtonian mechanics principle of conservation of angular momentum and the application in the quantum mechanical world.
CK-12 Foundation
Ck 12: Evolution of the Atomic Model
[Free Registration/Login may be required to access all resource tools.] Students take a closer look at how our understanding of the atom has evolved over time.
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Energy Skate Park
Explore kinetic, potential, and thermal energy as you send a skateboarder along several pre-built tracks, then design your own. Charts and graphs show the distribution of the different forms of energy, illustrating how the total energy...
CK-12 Foundation
Ck 12: Heat
[Free Registration/Login may be required to access all resource tools.] Using the law of conservation of energy as a starting off point, students use the specific heat equation to perform calculations that relate mass, specific heat,...
CK-12 Foundation
Ck 12: Chemical Equations
[Free Registration/Login may be required to access all resource tools.] Students explore mass relations between reactants and products for a given chemical process, and practice balancing chemical equations.