Curated OER
Drops on a Penny
Eighth graders experiment to determine the number of drops of water a heads up penny hold. They create a stem and leaf graph of the class results and use the data for further experimentation on a tails up penny.
Curated OER
Uranium On a Diet
Students examine how nuclear reactions differ from other chemical reactions they have studied. They add up atomic masses of both the product and reactant side of an example of uranium decomposition, demonstrating mass loss involved.
Curated OER
Science Quiz-General Knowledge
In this science quiz activity, students complete a set of 20 true or false questions covering a variety of general science topics. An answer key is included.
Curated OER
Hang a Million
Students hang 200 sheets of paper, each with 5000 X's, around the classroom. When completed, this gives them a concrete example of 1,000,000 which will be useful in getting a feel for the lengths of time involved in evolution.
Curated OER
Radioactive Decay/Half Life
Pupils demonstrate radioactive decay using candy corns. In this Physics lesson plan, students make predictions, collect data and form conclusions about patterns in radioactive decay.
Curated OER
Science-Introduction to the Unit on Matter
First graders gain knowledge about solids, liquids and gases by taking a look at the things around them. They have to classify certain picture cards based on which category they feel the picture falls under. As a whole, the class...
Curated OER
Where's the Evidence?
Eighth graders observe evidence of chemical reactions. In this chemical reactions instructional activity students study chemical processes and complete a lab activity.
Curated OER
Physical and Chemical Changes
Sixth graders complete several experiments about chemical and physical changes. In this physical and chemical science instructional activity, 6th graders complete 6 experiments about chemical and physical changes. Students examine the...
Curated OER
Calculating the Average Mass of the Newly Discovered Element: Bean
Students determine the average mass of a new element using masses from three isotopes. In this chemistry lesson, students explain what an isotope is. They discuss their importance and uses.
Curated OER
Teaching Place Value
Utilizing mathematical literature and games are two excellent ways to introduce and teach place value.
Frostburg State University
General Chemistry Online: Atoms & Ions
This site from the General Chemistry Online of the Frostburg State University provides a review of the history of atomic theory, the discovery of the electron, and the discovery of the nucleus. Details on weighing atoms, ion charges,...
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: Electron Clouds and Energy Levels
An explanation of the different types of atomic orbitals, how they are filled according to the Pauli Exclusion Principle, and how many electrons can fit in each electron shell.
CK-12 Foundation
Ck 12: Plix: Balancing Chemical Equations
[Free Registration/Login Required] Before you can balance an equation, you need to count the number of each atom present in the reactants and products. Using the example that is shown here, apply the numbers and table to track how many...
Khan Academy
Khan Academy: Photoelectron Spectroscopy
An explanation of how photoelectron spectroscopy can be used to detect electron structures in atoms or molecules.
Friesian School
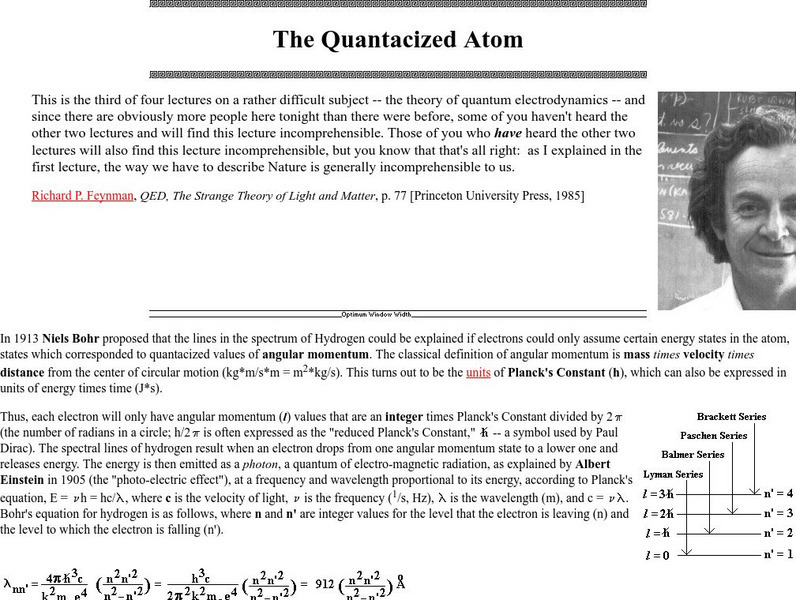
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Build a Molecule
Starting from atoms, see how many molecules you can build. Collect your molecules and see them in 3D.
CK-12 Foundation
Ck 12: Chemistry Simulation: Mole Carnival
[Free Registration/Login Required] In the mole carnival, students count the number of atoms and particles in different objects found at the carnival. As students work to find the number of particles, they practice counting particles by...
State University of New York
State University of New York: Determining Electron Pair Geometry
The electron-pair geometry of a molecule or ion depends on the number of structurally significant electron pairs in the central atom. Here students are asked to count the number of lone electron pairs and bonded atoms on the central...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Other popular searches
- Counting Atoms Molecules
- Counting Atoms in Compounds
- Counting Atoms Quiz
- Counting Atoms Coefficients
- Counting Atoms Worksheets