Khan Academy
Khan Academy: Dalton's Atomic Theory
Resource investigates the beliefs of Dalton's atomic theory which consists of four parts. Which points do we still use today, and what have we learned since Dalton?
Khan Academy
Khan Academy: Dalton's Atomic Theory
An article describing Dalton's atomic theory. Learn how this theory was the first attempt to describe a fundamental part of matter, atoms. Article also discusses where the theory is incorrect based on the knowledge we have today.
Towson University
Towson University: Ideal and Real Gas Laws
The ideal gas law is stated and explained at this site from the Towson University. It is then used to derive the other gas laws (Charles, Boyle's, Gay-Lussac's, Avogadro's, combined, etc.). Other gas law relationships are discussed....
Texas Education Agency
Texas Gateway: Temperature, Kinetic Theory, and the Gas Laws: Glossary
This is a glossary of terms and definitions used in Chapter 13: Temperature, Kinetic Theory, and the Gas Laws from the AP Physics online text.
Vision Learning
Visionlearning: Early Ideas About Matter: From Democritus to Dalton
Site that deals with the basics of matter. An interactive animation goes through experiments conducted in the 19th Century to help come up with scientific laws. With each section there are questions for comprehension.
University of Florida
Chemistry 2041 Lecture Notes: Ideal Gases
The ideal gas law is presented and explained. The derivation of other gas laws is performed. Gas behavior is explained in terms of gas laws. Excellent graphics.
Science Education Resource Center at Carleton College
Serc: Making Hydrogen Gas From a Decomposition Reaction
A very simple reaction of acid (HCl) and metal (magnesium) to produce Hydrogen gas and metal salt will provide the volume of gas to evaluate. Learners will determine the amount of gas produced at room conditions and then using the...
Science Education Resource Center at Carleton College
Serc:interactive Study: Laws of Conservation of Mass and Definite Proportions
Dalton's Playhouse is an interactive concept simulation meant to convey fundamental concepts in chemistry, including the law of definite proportions, the law of multiple proportions, and the law of conservation of mass.
Wolfram Research
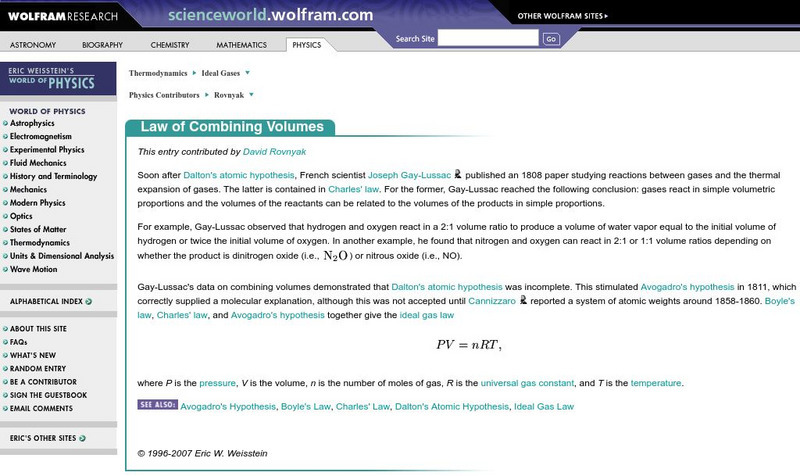
Wolfram Science World: Law of Combining Volumes
This site contains informaiton on the law of combining volumes. A formula is given and links to key terms.
CK-12 Foundation
Ck 12: Atomic Theory
[Free Registration/Login may be required to access all resource tools.] In this online tutorial students will explain the law of conservation of mass, the law of definite proportions, and the law of multiple proportions. They will also...
Simon Fraser University
Chem1 Virtual Textbook: Molar Mass of a Gas Mixture
The General Chemistry Virtual Textbook, or Chem 1, is broken into several sections covering various aspects of topics related to chemistry. This section deals with the molar mass of gas mixtures. Applications of Dalton's law are also...
Wyzant
Wyzant: Chem Tutor: Gases
A lengthy page covering most all the gas laws. Each law is described in words and stated as an equation. The use of each law in solving problems is demonstrated. Some problem-solving tips are provided. There are 23 practice problems with...
Michael Blaber, PhD
Florida State University: Gases: Gas Mixtures & Partial Pressure
Avogadro's law is stated in equation form. The lesson does not include much discussion, but it does relate the partial pressure and the total pressure of a gas sample to the mole fraction of its components.
Upper Canada District School Board
Tom Stretton's Advanced Placement Chemistry: Gases
This chemistry e-textbook provides students with AP-level reading and practice material on gas laws.
Vision Learning
Visionlearning: Chemical Relationships: Chemical Equations
An introduction to chemical reactions and an explanation of balanced equations.
Other
States of Matter and Properties of Gases: Terms
A very complete list of terms that are important to the study of gases. This resource is a web archive.
CK-12 Foundation
Ck 12: Evolution of the Atomic Model
[Free Registration/Login may be required to access all resource tools.] Students take a closer look at how our understanding of the atom has evolved over time.