Hi, what do you want to do?
Michael Blaber, PhD
Fsu: Basic Concepts of Chemical Bonding: Exceptions to the Octet Rule
Lists the exceptions to the octet rule and provides a discussion and diagrams explaining each one. Includes clear diagrams illustrating this concept.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Complete Electron Configuration
Test your knowledge of common atoms and their corresponding electron configurations. In this interactive exercise, you will find out how much you really know about the orbitals of electrons.
State University of New York
State University of New York: Electron Configurations
The following simulation provides an interaction with electron configurations.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: The Pauli Exclusion Principle
The Pauli Exclusion Principle shows how electrons fill atomic orbitals. Includes biographical information on Wolfgang Pauli.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: Quantum Numbers
Each electron has a set of quantum numbers that specify it's location, orbital, and energy in a unique manner.
Other
University of Texas: Tabled Discussion
At this University of Texas site, atomic orbital occupancy, quantum numbers, the Aufbau Principle, and periodic trends are described in detail.
CK-12 Foundation
Ck 12: The Quantum Mechanical Model
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will calculate the wavelength, frequency, and energy of light using Planck's constant and the speed of light. They will...
Chem4kids
Chem4 Kids: Helium
Here you can find lots of great information about helium. Learn about its electrons, where it exists in nature, and about the element itself!
Chem4kids
Chem4 Kids: Beryllium
Here at Chem4Kids you can find some great information about the 4th element in the periodic table, "beryllium." Content focuses on beryllium's electrons, where you can find beryllium in nature, and how beryllium combines with other...
Chem4kids
Chem4 Kids: Boron
Here at Chem4Kids.com you can find some great information about the 5th element in the periodic table, "boron." Content focuses on boron's electrons, where you can find boron in nature and in the home, and how boron combines with other...
Vision Learning
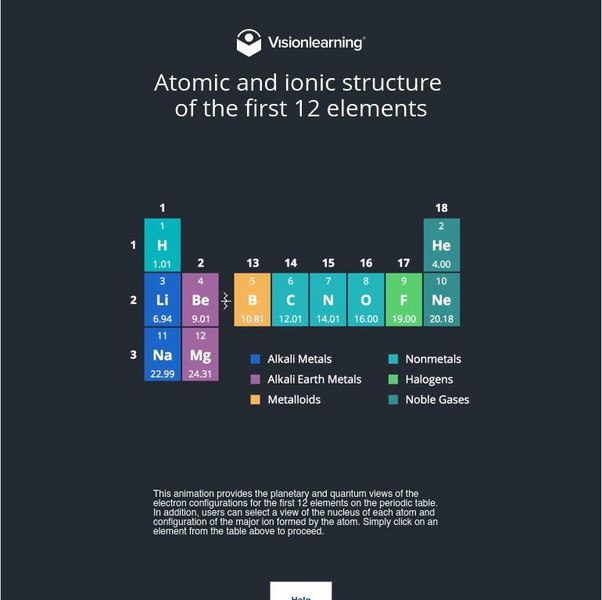
Visionlearning: Atomic and Ionic Structure of First 12 Elements
Analyze the electron configuration of the of the first twelve elements on the Periodic Table.
Other
Erik's Chemistry Page: Electronic Structure of Atoms
A description of quantum theory, the Bohr model of the atom, the quantum mechanical atom, the Scrodinger equation, and quantum numbers.
Other
Monterey Peninsula College: Pi Bond
Monterey Peninsula College faculty page simply shows a 3-D image of a Pi bonding orbital. Requires the CHIME plug-in
Purdue University
Purdue University: The Aufbau Principle
At this site from Purdue University, Electron Configurations, the Aufbau Principle, Degenerate Orbitals, and Hund's Rule are detailed. Includes learning exercises and answers.
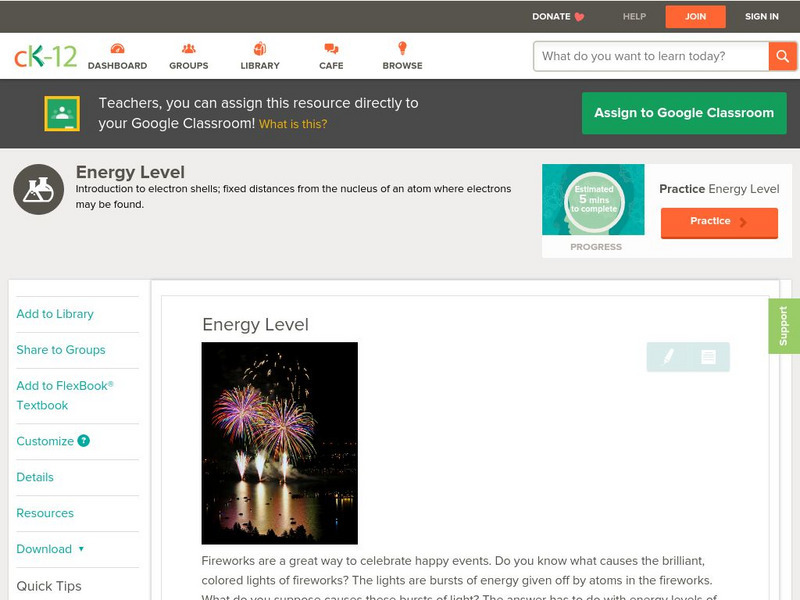
CK-12 Foundation
Ck 12: Physical Science: Energy Level
[Free Registration/Login may be required to access all resource tools.] Covers energy levels of atoms, their relation to orbitals, and the significance of electrons at different energy levels.
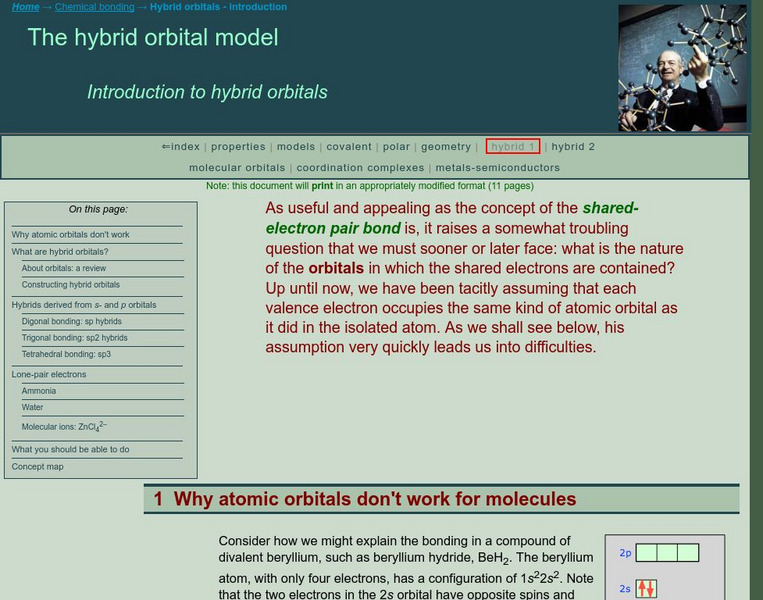
Simon Fraser University
Chem1 Virtual Textbook: The Valence Bond Model
The General Chemistry Virtual Textbook, or Chem 1, is broken into several sections covering various aspects of topics related to chemistry. This section deals with hybrid orbitals and specifically the valence bond model.
Davidson College
Davidson College: Effective Nuclear Charge
Explains what the effective nuclear charge is and how electrons can create a shield between a nucleus and an outer electron. Presents exercises for comparing orbital sizes with effective nuclear charges. Requires Java.
Oklahoma State University
Osu: Chemical Bonding Content in a Nutshell
An overview of chemical bonding and how valence electrons play a major role in these processes.
Michigan State University
Michigan State University: Elementary Physics Ii: The Pauli Exclusion Principle
The Pauli Exclusion Principle requires that all electrons in an atom have unique energy levels.
Chem4kids
Chem4 Kids: Lithium
Here at Chem4Kids you can find some great information about the third element in the periodic table, lithium. Content focuses on lithium's electrons, where you can find lithium in nature, and how it bonds with other elements (or with...
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Abbreviated Electron Configurations Help
This section from the online textbook "An Introduction to Chemistry" provides helpful hints to writing abbreviated electron configurations. Also learn how to look up the atom's outer shell electrons.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Elements With Unexpected Electron Configurations
This section of the online textbook "An Introduction to Chemistry", shows the unexpected electron configurations of six elements. A chart compares their predicted configurations with their actual configurations. Also find links to...
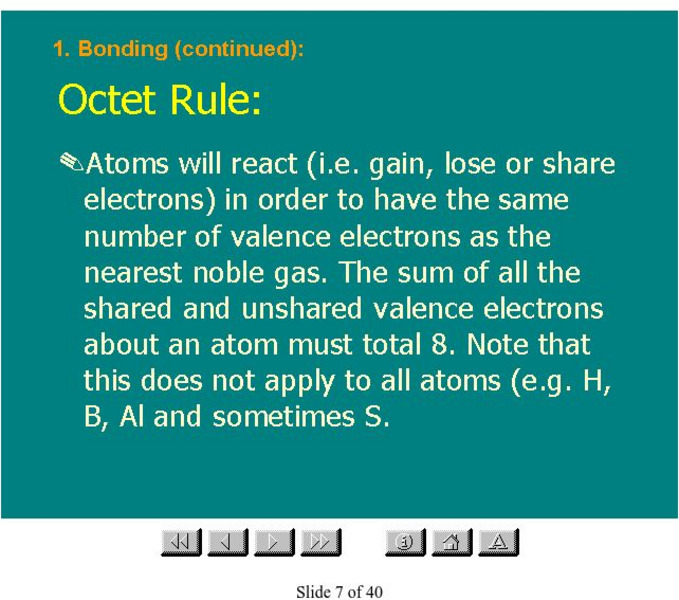
Other
Organic Chemistry: Octet Rule
This slide presentation contains a description of the octet rule, as well as many related topics.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Modern Atomic Theory [Pdf]
The modern atomic theory chapter from "An Introduction to Chemistry", describes electrons, electron orbitals, and how to draw orbital diagrams. Many pictures and examples help to explain the information. Also find a detailed chapter...