Hi, what do you want to do?
TeachEngineering
Teach Engineering: Antimatter Matters
Antimatter, the charge reversed equivalent of matter, has captured the imaginations of science fiction fans for years as a perfectly efficient form of energy. While normal matter consists of atoms with negatively charged electrons...
University of Maryland
Um: Quantum Mechanics of One and Two Electron Atoms
An explanation of Hund's rule and how to apply it to quantum mechanics. A mathematical-based explanation.
Khan Academy
Khan Academy: The Quantum Mechanical Model of the Atom
An explanation using quantum mechanics to describe the atom.
Vision Learning
Visionlearning: Atomic Theory: Bohr and the Beginnings of Quantum Theory
Description of the ideas and experimentation that led to quantum theory. Focus on electrically charged ions and isotopes.
University of Colorado
University of Colorado: Physics 2000: Periodic Table: Valences and the Periodic Table
The periodic table was laid out using valences or the electrons in the outermost shell.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: Spin
A basic explanation of spin and how it relates to electrons and quantum numbers.
Oklahoma State University
Oklahoma State University: Key Questions
A quiz on chemical bonding and valence electrons.
Virginia Tech
University of Vermont: Quantum Numbers
This chart highlights the characteristics of the first through fourth electron orbitals in quantum numbers. Also includes information about the Pauli Exclusion Principle.
Chem4kids
Chem4 Kids: Atoms
This site provides a detailed overview of atoms. Content explores an atom's structure, as well as what ions are, how atoms bond, what compounds are (including how to name compounds), and what isotopes are.
Georgia State University
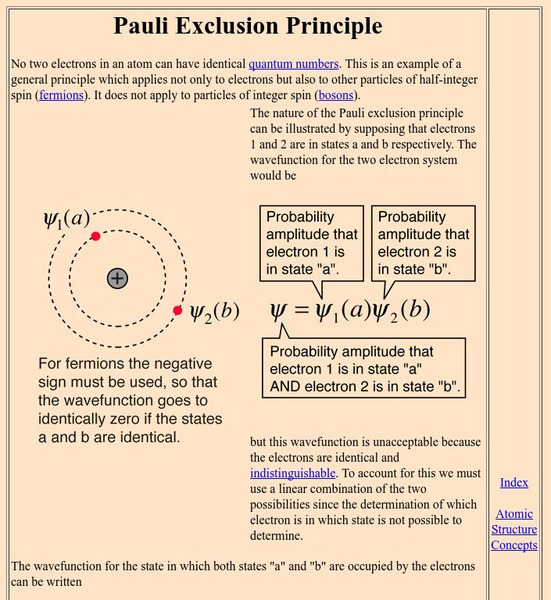
Georgia State University: Hyper Physics: Pauli Exclusion Principle
An explanation of the Pauli Exclusion Principle in mathematical terms.
Chem4kids
Chem4 Kids: Hydrogen
Here you can find out about hydrogen, the first element in the periodic table. Content includes shell information, where to find it in nature, and why it is helpful to us.
Environmental Chemistry
Environmental chemistry.com: Anatomy of an Atom
Explains the basics of atomic structure, from simple definitions to information about quantum theory. Accurate and helpful basics whether or not you need the more advanced information.
Simon Fraser University
Chem1 Virtual Textbook: The Aufbau Rules
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses atomic electron configurations. Specifically, the Aufbau rules are discussed with information on energies of...
CK-12 Foundation
Ck 12: Chemistry: Transition Metal Ions
[Free Registration/Login may be required to access all resource tools.] Covers transition metal, ionization of transition metals, and inner shell electrons.
CK-12 Foundation
Ck 12: Modern Atomic Theory
[Free Registration/Login may be required to access all resource tools.] Rutherford's model of the atom was better than earlier models. But it wasn't the last word. Danish physicist Niels Bohr created a more accurate and useful model....
Georgia State University
Georgia State University: Hyper Physics: Hund's Rules
A complete overview of Hund's rules including exceptions.
Georgia State University
Georgia State University: Hyper Physics: Hund's Rules
An excellent overview of Hund's Rules. Discusses all three rules thoroughly with provided diagrams.
Environmental Chemistry
Periodic Table of Elements: Gallium
A very detailed look at the element Gallium, a member of the Boron Group.
Environmental Chemistry
Periodic Table of Elements: Indium
A very detailed look at the element Indium, a member of the Boron Group.
Environmental Chemistry
Periodic Table of Elements: Thallium
A very detailed look at the element Thallium, a member of the Boron Group.
Purdue University
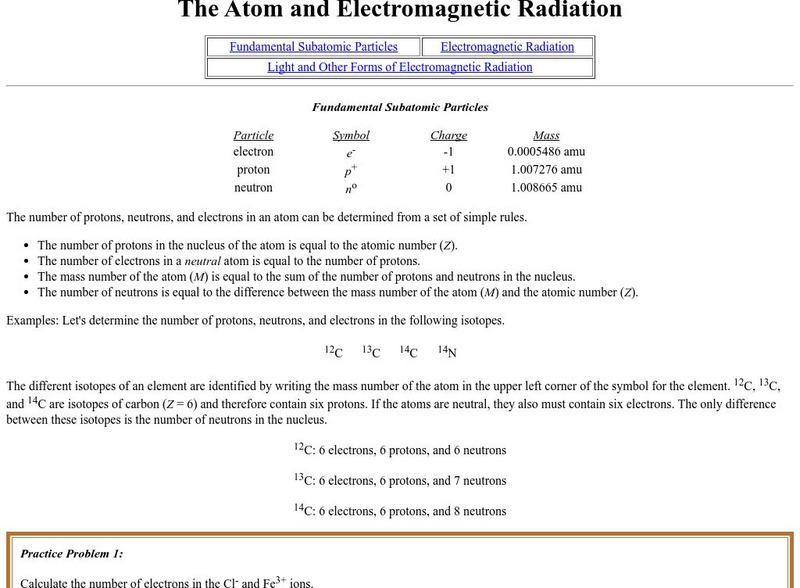
Purdue University: Fundamental Subatomic Particles
At this site from the Purdue University, the elementary subatomic particles are described and electromagnetic radiation is detailed. Includes learning exercises and answers.
Sophia Learning
Sophia: Octet Rule: Lesson 4
This lesson will define the Octet Rule and how this effects an atom's stability. It is 4 of 5 in the series titled "Octet Rule."
University of Colorado
University of Colorado: Physics 2000: Energy Levels
This site describes the energy levels of atoms.
Other
Chemtopics: Understanding the Schrodinger Eqn.
The Schrodinger equation specifies atomic orbitals which are occupied by an electron. Quantum numbers can identify a unique energy level for each electron (set up in a PowerPoint presentation).