Wolfram Research
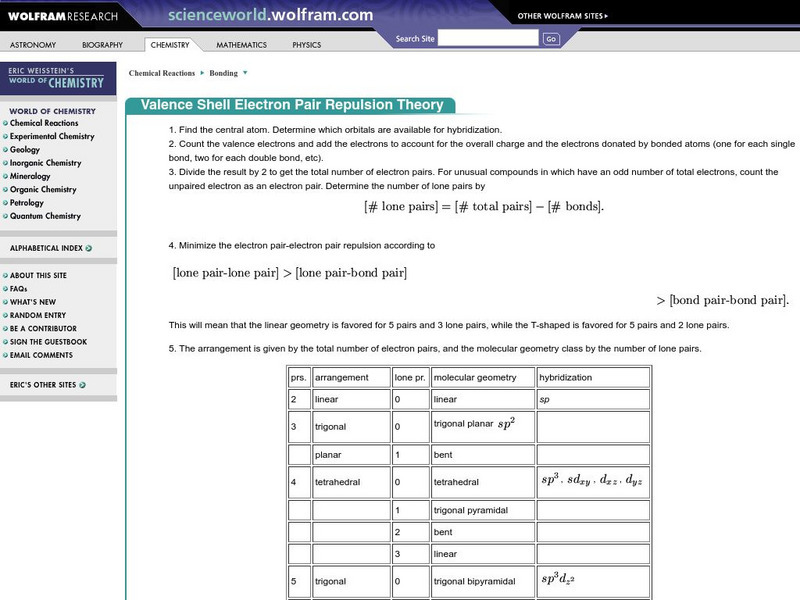
Wolfram Science World: Valence Shell Electron Pair Repulsion
Good site includes the basics of arrangement, hybridization, and gemetry.
CK-12 Foundation
Ck 12: Molecular Geometry
[Free Registration/Login may be required to access all resource tools.] The following online tutorial explains the basis of VSEPR theory. It helps students predict the shapes of molecules and polyatomic ions using VSEPR theory and it...
Simon Fraser University
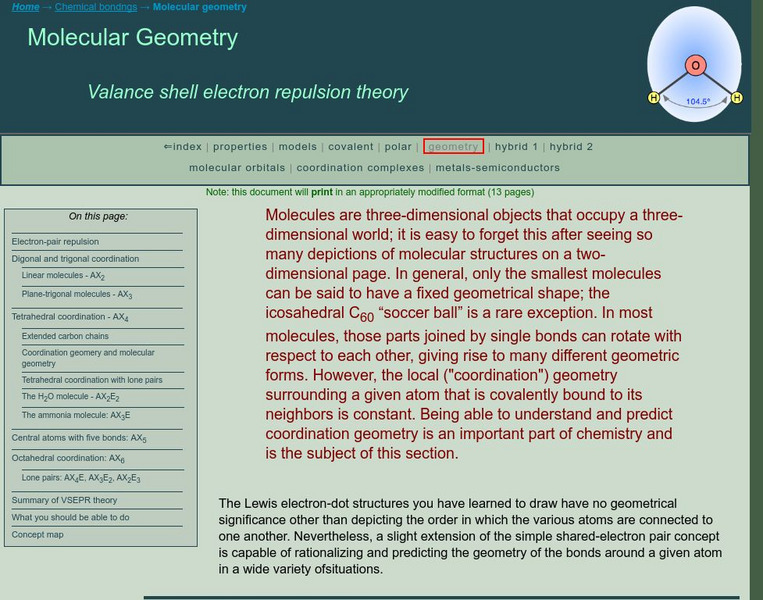
Chem1 Virtual Textbook: Molecular Geometry
An advanced explanation of the valence shell electron pair repulsion (VSEPR) theory describes specific molecular models involving digonal, trigonal, tetrahedral, and octahedral coordination, as well as central atoms with five bonds....
CK-12 Foundation
Ck 12: Molecular Geometry
[Free Registration/Login may be required to access all resource tools.] In this interactive learning module, students will learn a technique to predict molecular geometry based on a molecule's Lewis electron dot structure.
University of Waterloo (Canada)
University of Waterloo: Vsepr Models
The University of Waterloo provides background in pair repulsion and confidence building questions. Try this one once you think you know what you are doing!
State University of New York
State University of New York: Effective Nuclear Charge, Z*
The following simulation provides an explanation of effective nulcear charge, Z*.
Concord Consortium
The Concord Consortium: Molecular Workbench: Electrical Charge Maze
Position positive and negative charges to help an electron move through a maze to hit a target.
University of Colorado
University of Colorado: Ph Et Interactive Simulations: John Travoltage
See how electrons build up and are discharged as static electricity.
Science4Fun
Science4 Fun: Magnetism
What is magnetism? Learn about magnetic domains, how magnets attract or repel, magnetic materials, electromagnets and their uses, and the history of magnets.
PBS
Pbs Learning Media: Molecular Shapes
In this interactive activity from ChemThink, students will learn about covalent molecules and how the VSEPR theory predicts the shapes of covalently-bonded molecules.