American Chemical Society
Middle School Chemistry: Energy Levels, Electrons, Ionic Bonding
Students discover that ionic bonding occurs when electrons are transferred from one atom to the other and not shared as in covalent bonding.
American Chemical Society
Middle School Chemistry: Chapter 4: The Periodic Table and Bonding
Six middle school chemistry lessons about the periodic table and bonding complete with handouts and animations.
University of Southern California
Atomic Bonds
This slide show on atomic bonds contains several slides on electron affinity. Other topics include covalent, Sigma and Pi bonds, and atomic bonding in solids.
Science Education Resource Center at Carleton College
Serc: Valence Electrons and Trends in the Periodic Table
This instructor led activity will produce a partially filled periodic table that contains electron-dot models for the first twenty elements in the appropriate boxes. It will be used as a visual tool for students to connect concepts such...
Other
Chemical Bonds: Formal Charges
A slide presentation with several slides dedicated to the topic of formal charges. Formal charges are explained, and examples are given. A practice example is provided.
Wisc-Online
Wisc Online: Lewis Dot Structures of Covalent Compounds
Short slide show provides basic information about drawing Lewis dot structures for covalent compounds. Starts with anatomy of the atom, and then shows the relationship between atomic particles and the Periodic Table of Elements. Offers...
Sophia Learning
Sophia: Characteristics of Chemical Bonds: Lesson 4
This lesson will present the basic properties and characteristics of chemical bonds. It is 4 of 4 in the series titled "Characteristics of Chemical Bonds."
Utah Education Network
Uen: Grouping Bonds
Students will group a selection of electron dot structures and determine similarities and differences between covalent and ionic bonding.
McMaster University
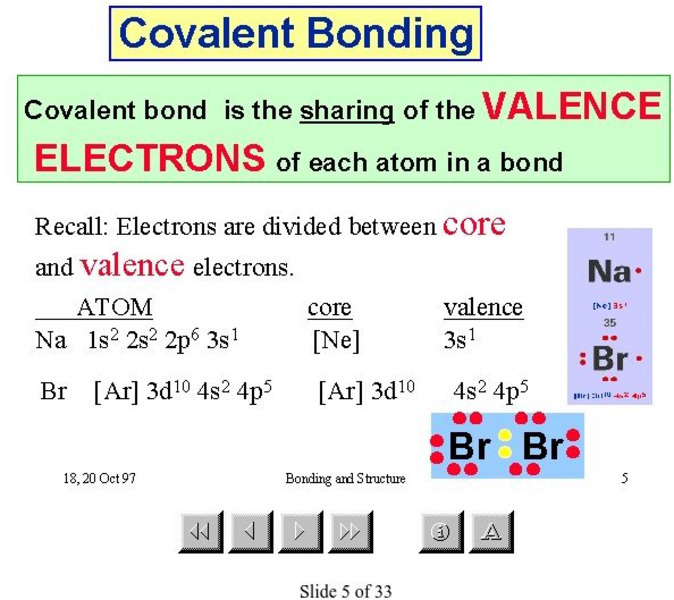
Mc Master University: Covalent Bonding
Slides 5 through 8 in this presentation from the McMaster University explain covalent bonding.
Other
Organic Chemistry: Lewis/kekule Structures
This slide presentation contains a few slides that will be useful for students learning to write Lewis structures. A discussion of multiple bonds is included, and clear examples are shown.
Other
Chemical Bonds: Writing Lewis Structures
This slide show explains the rules for writing Lewis structures and then demonstrates an example. It then provides a practice example for the student to try.
American Chemical Society
Middle School Chemistry: Represent Bonding With Lewis Dot Diagrams
Learners draw and interpret Lewis dot diagrams for individual atoms and both covalent and ionic compounds.
Science Education Resource Center at Carleton College
Serc: Loopy Lewis Dot Diagrams
Students will use colored fruit loops to organize valence electrons to develop and master the basics of Lewis Dot diagrams. They will develop processing and critical thinking skills and also master a model of bonding.
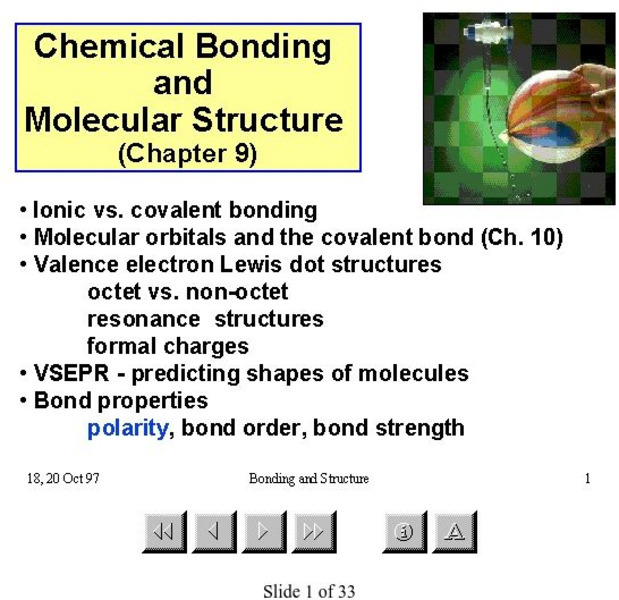
McMaster University
Mc Master University: Molecular Structure
This PowerPoint presentation features 33 slides that explain chemical bonding and molecular shape.
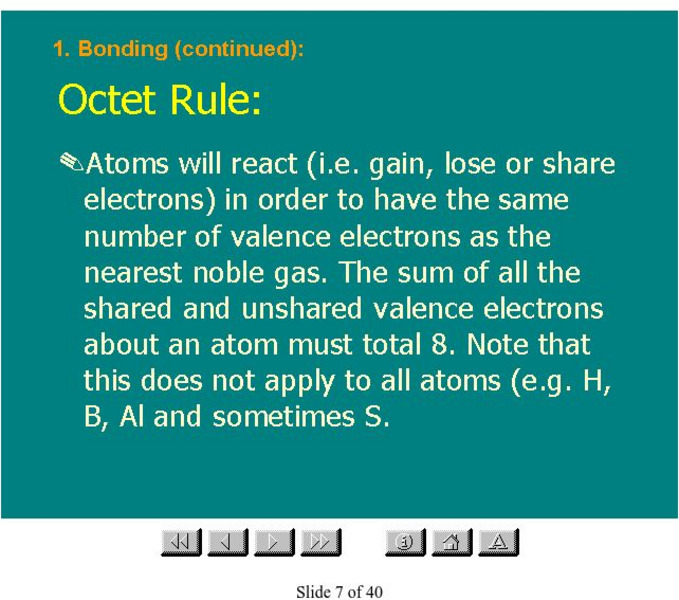
Other
Organic Chemistry: Octet Rule
This slide presentation contains a description of the octet rule, as well as many related topics.
American Chemical Society
Middle School Chemistry: Lesson Plans: Water Is a Polar Molecule
Young scholars develop their own water molecule model to help them understand the idea that water has a slight positive charge at one end of the molecule and a slight negative charge at the other.