Hi, what do you want to do?
Purdue University
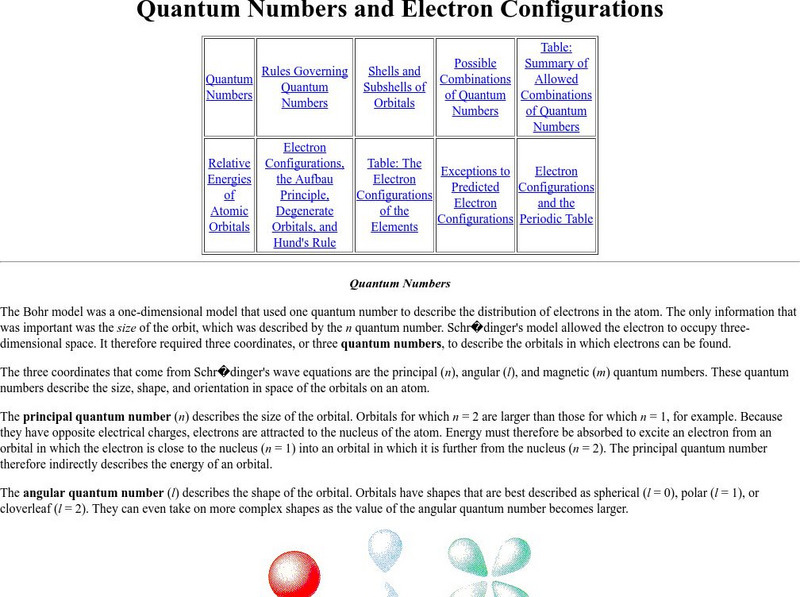
Purdue University: The Aufbau Principle
At this site from Purdue University, Electron Configurations, the Aufbau Principle, Degenerate Orbitals, and Hund's Rule are detailed. Includes learning exercises and answers.
Georgia Department of Education
Ga Virtual Learning: Ap Chemistry: Atomic Theory
In this module students explore how matter is classified, the history of atomic theory, subatomic particles, modern atomic theory, electron configuration, the periodic table and its trends, and spectroscopy.
Vision Learning
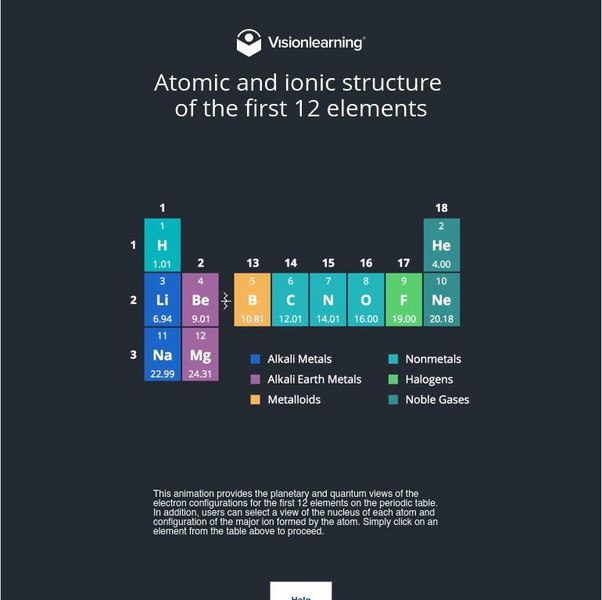
Visionlearning: Atomic and Ionic Structure of First 12 Elements
Analyze the electron configuration of the of the first twelve elements on the Periodic Table.
Vision Learning
Visionlearning: Atomic Theory and Structure: The Periodic Table
A description of the arrangement of elements on The Periodic Table based on electron configuration.
Chem Tutor
Chem tutor.com: Atomic Structure
This page provides a thorough and comprehensible discussion of the structure of the atom. Includes helpful diagrams and instructions for filling electron shells, reading the Periodic Table, and drawing Lewis Structures.
University of Colorado
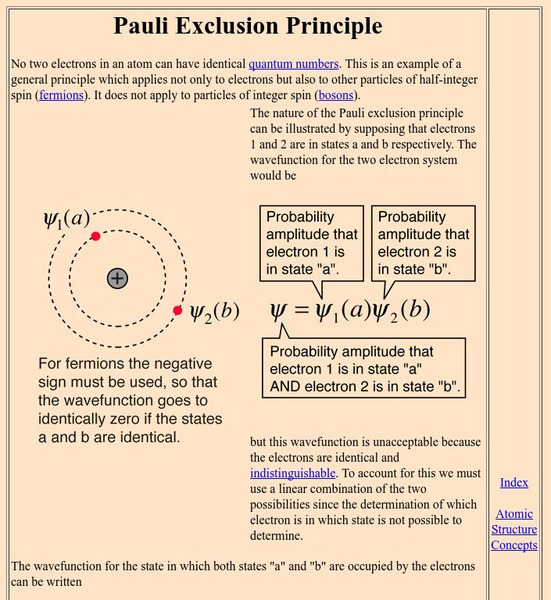
University of Colorado: Physics 2000: Elements as Atoms: The Pauli Exclusion Principle
The Pauli Exclusion Principle shows how electrons fill atomic orbitals. Includes biographical information on Wolfgang Pauli.
University of Colorado
University of Colorado: Physics 2000: Elements as Atoms: Quantum Numbers
Each electron has a set of quantum numbers that specify it's location, orbital, and energy in a unique manner.
Other
Web Chem: Bonding: Valence and Core Electrons
This site from WebChem contains information on the subject of bonding with an emphasis on valence and core electrons.
BBC
Bbc: Gcse Bitesize: The Periodic Table
This lesson focuses on the periodic table and the configurations. In the modern periodic table, elements are in order of atomic number in periods and groups. Electronic configurations model how electrons are arranged in atoms. A link to...
Simon Fraser University
Chem1 Virtual Textbook: The Aufbau Rules
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses atomic electron configurations. Specifically, the Aufbau rules are discussed with information on energies of...
CK-12 Foundation
Ck 12: Chemistry: Transition Metals
[Free Registration/Login may be required to access all resource tools.] Covers electron configurations of transition elements.
Other
Erik's Chemistry Page: Electronic Structure of Atoms
A description of quantum theory, the Bohr model of the atom, the quantum mechanical atom, the Scrodinger equation, and quantum numbers.
PBS
Pbs Learning Media: Periodic Table of the Elements
This interactive periodic table developed for Teachers' Domain provides detailed information about the chemical properties of elements and illustrates the electron configurations that determine those characteristics.
Oklahoma State University
Oklahoma State University: Ionization Energy Trends
Site that discusses trends in ionization energy across and down the periodic table. Apparent contradictions are explained using electron configurations.
BBC
Bbc: Gcse Bitesize: What Does the Periodic Table Tell Us About the Elements?
The number of protons in the atom of an element determines its place in the Periodic Table. The number of electrons in an atom is the same as the number of protons. These electrons are arranged in shells or 'energy levels' around the...
Michigan State University
Michigan State University: Elementary Physics Ii: The Pauli Exclusion Principle
The Pauli Exclusion Principle requires that all electrons in an atom have unique energy levels.
University of Maryland
Um: Quantum Mechanics of One and Two Electron Atoms
An explanation of Hund's rule and how to apply it to quantum mechanics. A mathematical-based explanation.
Khan Academy
Khan Academy: Photoelectron Spectroscopy
An explanation of how photoelectron spectroscopy can be used to detect electron structures in atoms or molecules.
CK-12 Foundation
Ck 12: Chemistry: Hydrogen and Alkali Metals
[Free Registration/Login may be required to access all resource tools.] Covers Group I elements, electron configuration, and reactivity.
Georgia State University
Georgia State University: Hyper Physics: Pauli Exclusion Principle
An explanation of the Pauli Exclusion Principle in mathematical terms.
Other
University of Texas: Tabled Discussion
At this University of Texas site, atomic orbital occupancy, quantum numbers, the Aufbau Principle, and periodic trends are described in detail.
Science Struck
Science Struck: Ground State vs. Excited State of an Atom
Explains what Bohr's model of the atom is, the characteristics of the ground and excited states of an atom, and the electron configuration for each in the example of Phosphorus.
Concord Consortium
Concord Consortium: Molecular Workbench: Atomic Orbitals
View the approximate locations of electrons in valence shells 1s through 4f.
University of Colorado
University of Colorado: Physics 2000: Periodic Table: Valences and the Periodic Table
The periodic table was laid out using valences or the electrons in the outermost shell.