Hi, what do you want to do?
Curated OER
Units in Thermochemical Calculations
Young scholars study how to complete thermochemical equations. In this equation lesson students learn how to manipulate equations to calculate energy changes and reactions.
Curated OER
Endothermic or Exothermic Reactions
In this chemical reaction worksheet, students determine if given reactions are endothermic or exothermic reactions. Students determine the energy released or consumed when chemical reactions occur. This worksheet has 5 problems to solve.
Creative Chemistry
Trends in Physical Properties of Group 2 Elements
In this elements learning exercise, learners complete two graphic organizers by comparing the element symbol and melting point for given elements. Then students plot a graph of their atomic radius against proton number.
Curated OER
Chemistry Practice
In this chemistry practice activity, students select the correct response to the given questions. Students apply knowledge about the states of matter, finding vapor pressure and atmospheric pressure.
Curated OER
Thermodynamics Worksheet
In this science worksheet, students are using the laws of thermodynamics and a basic knowledge to fill in the sentences with the correct words.
Curated OER
Thermochemistry Calculations
In this chemistry worksheet, students apply reaction equation ratios to solve fifteen problems, including five using a specific formula provided on the worksheet.
Dartmouth College
Dartmouth College: Chem Lab: Calorimetry 1 Enthalpy of Formation of Mg O
In this lab experiment students are asked to determine the standard molar enthalpy of formation of magnesium oxide, MgO. Students are asked to use Hess's Law and the enthalpy changes of several other solution reactions during this...
CK-12 Foundation
Ck 12: Heat and Changes of State
[Free Registration/Login may be required to access all resource tools.] Students explore the enthalpy changes that occur as substances change between states, and then calculate the enthalpy change involved in the change of state.
CK-12 Foundation
Ck 12: Hess's Law and Standard Enthalpy of Formation
[Free Registration/Login may be required to access all resource tools.] Students will use Hess's law of heat summation to add chemical reactions together to produce a desired final equation, and then calculate the enthalpy change for...
Science Education Resource Center at Carleton College
Serc: Energy Changes During a Reaction: Exothermic, Endothermic
A demonstration to introduce energy changes during reactions, and to promote discussion regarding exothermic and endothermic reactions, enthalpy, spontaneity, system, and surroundings.
Frostburg State University
General Chemistry Online: Energy and Chemical Change Faq
Check out answers to many common questions about energy and its relationship to chemical change. Understand details in topics such as enthalpy and thermochemistry with these FAQ.
BBC
Bbc: Gcse Bitesize: Heat Energy Changes in Chemical Reactions
This lesson focuses on heat energy changes in chemical reactions. Exothermic reactions transfer energy to the surroundings. Endothermic reactions take in energy from the surroundings. It also offers links to a video and a test.
Shodor Education Foundation
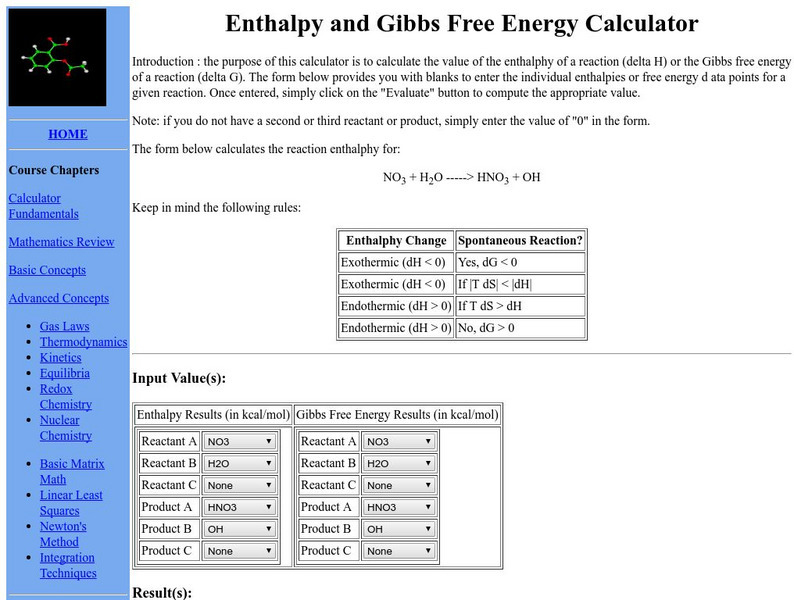
Shodor: Enthalpy and Gibbs Free Energy Calculator
Interactive calculator that allows you to input reactants and products of a reaction and calculate the change in enthalpy and Gibbs Free Energy for the reaction.
CK-12 Foundation
Ck 12: Thermochemical Equations
[Free Registration/Login may be required to access all resource tools.] Students investigate the conditions under which the enthalpy change in a reaction is equal to the heat absorbed or released. Then, they have the opportunity to write...
CK-12 Foundation
Ck 12: Plix Series: Enthalpy
[Free Registration/Login Required] Move the chemical reaction along, and observe the overall change in enthalpy for each reaction in the simulation. After the activity, answer one challenge question to check for understanding.
Texas Instruments
Texas Instruments: Designing Hot and Cold Packs
in this activity, students calculate the molar enthalpy of dissolving for three ionic solids, and use this information to design a cold pack. In this experiment they measure the heat changes which occur from various salts dissolving in...
Khan Academy
Khan Academy: Test Prep: Mcat: Chemical Processes: Thermochemistry: Endothermic vs. Exothermic Reactions
Using an illustrated example, the difference between an endothermic and an exothermic reaction is described. The concept of enthalpy, or heat energy change, is explained, and the energy diagrams of reactions are presented.
Other
University of Siegen: Enthalpy of Solution
This resource provides a physical reaction. Hydrous and anhydrous calcium chloride react with water to produce physical exothermic and endothermic reactions.
CK-12 Foundation
Ck 12: Spontaneous Reactions and Free Energy
[Free Registration/Login may be required to access all resource tools.] Students discover the concept of a spontaneous reaction in terms of enthalpy and entropy changes, and then investigate and calculate free energy.
Chemistry Collective
Chem Collective: Camping Problem Ii
In this part of the MRE scenario, students determine change in the enthalpy of a reaction as the concentration of reactants are varied.
TeachEngineering
Teach Engineering: Counting Calories
The students discover the basics of heat transfer in this activity by constructing a constant pressure calorimeter to determine the heat of solution of potassium chloride in water. They first predict the amount of heat consumed by the...
CK-12 Foundation
Ck 12: Free Energy and Equilibrium
[Free Registration/Login may be required to access all resource tools.] Students determine the temperature at which a reversible reaction will achieve equilibrium by using the Gibbs free energy equation, and then describe the...
Other
Matter Project: What Is Bond Energy?
Provides a Java applet that shows the change in energy of a bonded and a non-bonded atom.
Savvas Learning
Chemistry: Central Science Live: Properties of Solutions
This site provides an excellent overview of the solution process. Content includes a focus on how a solution is formed, energy changes and solution formation, spontaneity and disorder, and solution formation and chemical reactions.