Hi, what do you want to do?
Curated OER
Toothpick Sculpture
You'll be surprized at how much engineering, design, and creativity can go into a toothpick and a marshmallow. Learners make toothpick sculptures where they construct three-dimentional models by sticking marshmallows and toothpicks...
Curated OER
Flambe Elements
Eighth graders discuss atoms and electrons as well as atoic structure. They view atomic structure via the computer. Students watch a demonstration in which the teacher demonstrates glass tubing turning yellow in a Bunsen Burner flame....
Curated OER
Atoms : A Self Guided Computer Activity
"Self-guided Computer Activity" simply means that young chemists read through the slides and take notes about atoms along the way. There is an abundance of text on each slide, making this a comprehensive introduction to atomic structure....
Curated OER
Electromagnetic Spectrum/Spectroscopy
Students examine the electromagnetic spectrum and demonstrate the elements within. In this investigative lesson plan students complete a demonstration and calculate the energy of a photon.
Curated OER
The Flame and the Atom:
Learners investigate the structure of atoms. Students read information about the Rutherford model, the Bohr model, and the Quantum Mechanical model examining each for its scientific validity. They watch a PowerPoint presentation of alpha...
Curated OER
Build Your Own Atom
Young scholars build a model of an atom using an online program. In this chemistry lesson, students discuss the different parts of the atom. They complete an independent research about their chosen element.
Curated OER
Activity One -- Fundamentally Speaking
For this science worksheet, students examine how the universe is bound with the atomic particles and focus upon the findings of two historical scientists.
Curated OER
How Can You Study Things You Can’t See Like: Atoms?
Students simulate how scientists studied things they can't see like atoms. In this chemistry lesson, students predict what is inside the numbered obsertainers. They design a way to investigate what's inside without opening it.
Other
Universe Today; What Is John Dalton's Atomic Model?
Article explaining the legacy of John Dalton. His research into gases led to his gas laws and then his proposed atomic model. The flaws in his atomic theory are discussed also. (Jan. 20, 2016)
Tom Richey
Slide Share: Atomic Models: Everything You Need to Know
A detailed slideshow for Grade 8 students that looks at the history of our understanding of atoms. Looks at the ideas presented by Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr, and the modern-day wave model. A...
Texas Education Agency
Texas Gateway: Atoms, Elements and the Periodic Table: The Atomic Model [Pdf]
A slideshow looking at the contributions of scientists over time to our understanding of atomic theory. Looks at models of the atom developed by Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick, as...
Other
Crocodile Clips: Absorb Chemistry: History of the Atom
A tutorial that presents models of the atom proposed by John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr. Each is supported by an animated illustration. Includes comprehension questions and a quiz at the end.
Other
A Short History of the Atom
A classroom wiki where students present profiles of scientists who developed models of the atom or who contributed to the understanding of atomic theory. Covers Democritus, Aristotle, John Dalton, J.J. Thomson, Ernest Rutherford, Marie...
Tom Richey
Slide Share: Atomic Structure
Slideshow looking at the history of models of the atom, including those proposed by John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick. Discusses subatomic particles, including the numbers of protons, neutrons,...
Other
Slide Player: History of the Atomic Model
Short slideshow looking at the history of models of the atom, including the contributions of Aristotle, Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr.
Science Struck
Science Struck: Plum Pudding Model
Describes J.J. Thomson's theory of atomic structure, called the 'Plum Pudding Model,' and how it was disproved by Ernest Rutherford.
CK-12 Foundation
Ck 12: The Bohr Model of the Atom
[Free Registration/Login may be required to access all resource tools.] Students will learn about the history of atomic theory, and the development of the Bohr model of the atom. Includes a simulation for exploring the Bohr Model.
CK-12 Foundation
Ck 12: Modern Atomic Theory
[Free Registration/Login may be required to access all resource tools.] Rutherford's model of the atom was better than earlier models. But it wasn't the last word. Danish physicist Niels Bohr created a more accurate and useful model....
American Museum of Natural History
American Museum of Natural History: O Logy: Stuff to Do: Atomic Mobile
Illustrated instructions for how to make a model of an atom (an atom mobile).
Other
Cronodon: Atoms Models of the Atom
Presents models of the atom developed by Thomson, Rutherford, Bohr, and Schrodinger. Includes successes and failures of each, illustrations, and detailed descriptions. Also discusses Dirac's model of the atom and introduces quantum...
Texas Instruments
Texas Instruments: The History Behind the Atom
This StudyCard activity enables students to review the key contributions by scientists and philosophers towards are current understanding of the atom. It also allows students to review characteristics of the major atomic models.
Khan Academy
Khan Academy: Bohr's Model of Hydrogen
Resource investigates how Bohr's model of hydrogen explains atomic emission spectra.
Khan Academy
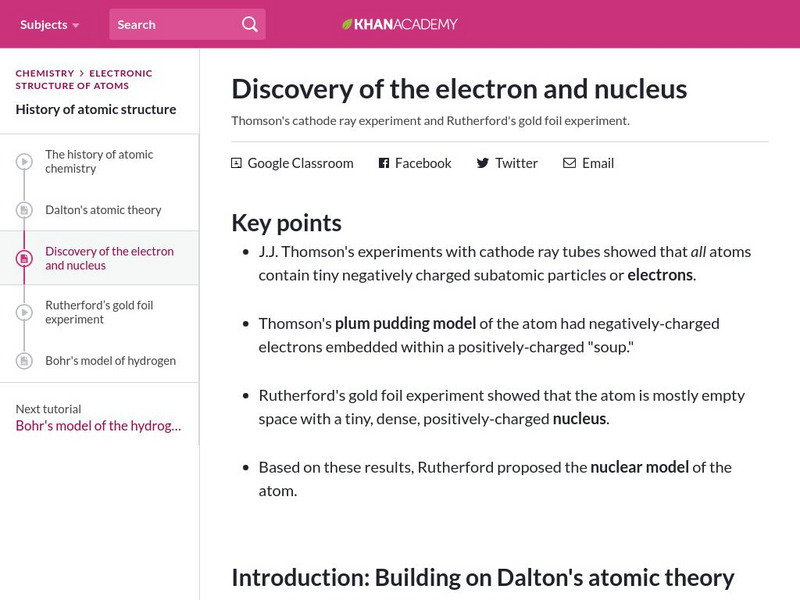
Khan Academy: Discovery of the Electron and Nucleus
Learn about Thomson's cathode ray experiment and the plum pudding model and Rutherford's gold foil experiment.
Boise State University
Boise State University: Atoms: A Virtual Field Trip Through Time and Space
Learn about the models of the atom that have been proposed throughout history. Presents Democritus, John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and the modern theory of the atom. Sections are accompanied by journal...