Science Education Resource Center at Carleton College
Serc: Models of the Hydrogen Atom
In this activity, students will explore several different models of the hydrogen atom and compare and contrast them using an online java applet.
Simon Fraser University
Chem1 Virtual Textbook: Spectrum of the Hydrogen Atom
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses the hydrogen atom and its relation to spectrum. Included in the discussion is information on the Bohr model...
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Models of the Hydrogen Atom
How did scientists figure out the structure of atoms without looking at them? Try out different models by shooting light at the atom. Check how the prediction of the model matches the experimental results.
Dartmouth College
Dartmouth College: Chem Lab: Spectrum of the Hydrogen Atom
In this experiment, you will use a meterstick spectroscope to observe the emission spectrums of hydrogen, sodium, neon, helium, and mercury. Requires Java plug-in.
Walter Fendt
Walter Fendt: Bohr's Theory of the Hydrogen Atom
An explanation of Niels Bohr's (1885 - 1962 CE) Model along with an app that illustrates a hydrogen atom according to the particle or wave model. You can choose a principal quantum number "n." The right part of the graphic represents the...
Michael Blaber, PhD
Florida State University: The Bohr Model of the Atom
A well designed clear tutorial explaining the energies involved in the Bohr model of the atom. Illustrations add to the clearly presented equations.
Other
Brockport High School: Energy Levels of Hydrogen Atom
From the Brockport High School Physics Labs web pages. Includes an excellent graphic depicting the energy levels of a hydrogen atoms and portraying the electron level transitions for the Lyman, Balmer, and Paschen series. Includes both...
Georgia State University
Georgia State University: Hyper Physics: Hydrogen Energies and Spectrum
This site from Georgia State University gives information on the transitions of electrons between energy levels. The energy levels for electrons in the hydrogen atom are discussed. The Rydberg equation is stated and electron transitions...
Other
Ap Physics Lab: Energy Levels of the Hydrogen Atom
A lab activity from an AP Physics course. Students measure the energy changes associated with electron level transitions to the second energy level for hydrogen gas. Includes directions and suggestions. Ideal for a student project or lab...
Simon Fraser University
Chem1 Virtual Textbook: The Bohr Atom
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses Niels Bohr and his work with the atom. Topics covered in the discussion include the atom before Bohr, Bohr's...
Khan Academy
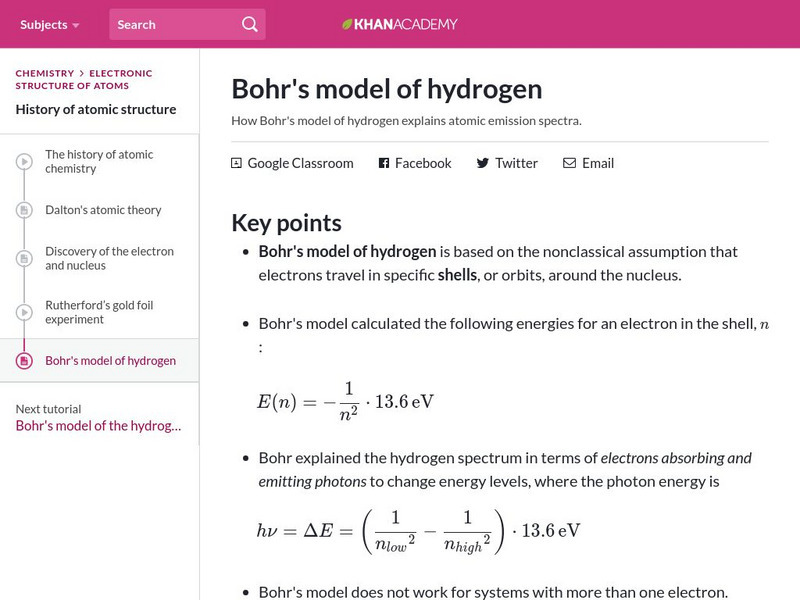
Khan Academy: Bohr's Model of Hydrogen
Resource investigates how Bohr's model of hydrogen explains atomic emission spectra.
Khan Academy
Khan Academy: Bohr's Model of Hydrogen
How Bohr's model of hydrogen explains atomic emission spectra.
Encyclopedia Britannica
Encyclopedia Britannica: Hydrogen Ion
This brief entry describes the hydrogen ion, strictly, as the nucleus of a hydrogen atom separated from its accompanying electron. The hydrogen nucleus is made up of a particle carrying a unit positive electric charge, called a proton.
University of Alberta
The University of Alberta: Nmr Spectroscopy
1HNMR theory begins in the nucleus of hydrogen. Complete this tutorial on the 1HNMR spectrum graph to learn about the number of equivalent hydrogens, b) the chemical environment of each hydrogen type and c) the number of neighbouring...
Simon Fraser University
Chem1 Virtual Textbook: The Quantum Numbers
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses quantum numbers of electrons in atoms including topics such as principal quantum number and orbitals of the...
University of Colorado
University of Colorado: Physics 2000: Bohr's Atom
An outstanding site that can best be described as student friendly. It describes the main differences between the classical and quantum models of the atom. Clear and well illustrated.
Friesian School
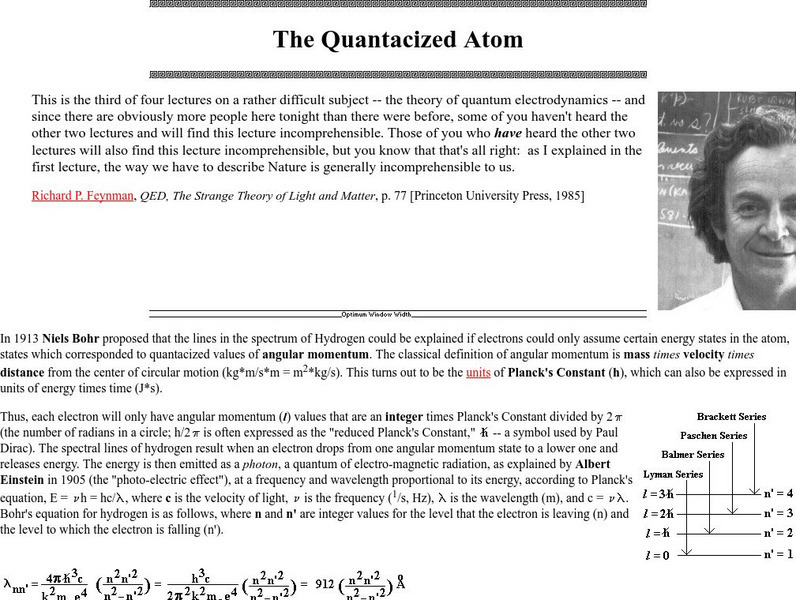
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Science Kids: Science Images: Hydrogen Atom
This is a simple picture of a hydrogen atom using the Bohr model. A negatively charged electron can be seen on the outside of the positively charged proton.
State University of New York
State University of New York: Atomic Absorption and Emission
This module simulates the excitation of hydrogen atoms through irradiation with electromagnetic radiation of different wavelengths.
Concord Consortium
Concord Consortium: Reaction Between Hydrogen and Oxygen Atoms
Explore energy during a reaction between hydrogen and oxygen atoms.
Other
Atom Central: The H Bomb & the Birth of the Universe
Peer into the heart of nuclear explosions through a series of photographs of the detonation of Hydrogen Bombs.
Ducksters
Ducksters: Chemistry for Kids: Elements: Hydrogen
On this website, the element hydrogen and its chemistry including atomic weight, atom, uses, sources, name, and discovery are discussed. Plus properties and characteristics of hydrogen.
CK-12 Foundation
Ck 12: The Bohr and Quantum Models of the Atom
[Free Registration/Login may be required to access all resource tools.] Students explore how the study of the hydrogen emission spectrum led to the Bohr model of the atom, in which electrons exist in states of constant energy.
Curated OER
Brockport High School: Energy Levels of Hydrogen Atom
From the Brockport High School Physics Labs web pages. Includes an excellent graphic depicting the energy levels of a hydrogen atoms and portraying the electron level transitions for the Lyman, Balmer, and Paschen series. Includes both...