Hi, what do you want to do?
TeachEngineering
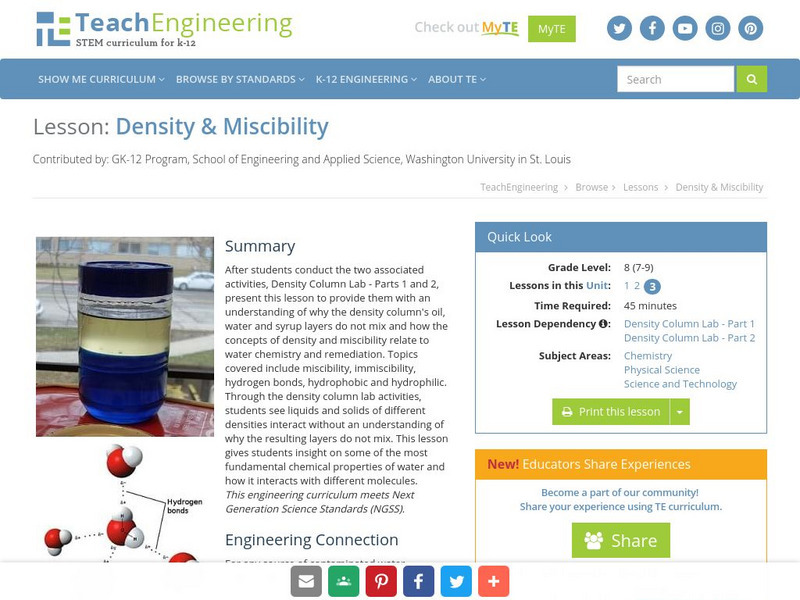
Teach Engineering: Density & Miscibility
After students conduct the two associated activities, Density Column Lab - Parts 1 and 2, present this lesson to provide them with an understanding of why the density column's oil, water and syrup layers do not mix and how the concepts...
Frostburg State University
General Chemistry: Why Oh, Nh, and Fh Bonds Are Polar
Frostburg State University provides a brief explanation to the question of why small hydrogen molecules, such as OH, NH, and FH, are very polar.
Other
What Is Life: Aqueous Solutions
Discussion of water as a solvent. Includes material on hydrogen bonds, hydration, hydrophobic effect, acids, basses, and pH.
American Chemical Society
Middle School Chemistry: Energy Levels, Electrons, and Covalent Bonding
Discover how covalent molecular bonding affects the energy levels of electrons.
John Wiley & Sons
Concepts in Biochemistry: Concept Reviews: Water, P H, and Non Covalent Bonding
A detailed review of water and its structure. Included are diagrams, animated graphics, and review quizzes. For the advanced high school student.
Curated OER
Science Kids: Science Images: Hydrogen Bonds
A computer generated image showing a 3D model of hydrogen bonds in water.
CK-12 Foundation
Ck 12: Chemistry: Physical Properties of Water
[Free Registration/Login may be required to access all resource tools.] Covers physical properties of water, hydrogen bonding, surface tension, and vapor pressure.
CK-12 Foundation
Ck 12: Chemistry: Structure of Water
[Free Registration/Login may be required to access all resource tools.] Covers structure of water molecule, hydrogen bonding on water, polarity of water molecule, and bond angles of water molecule.
CK-12 Foundation
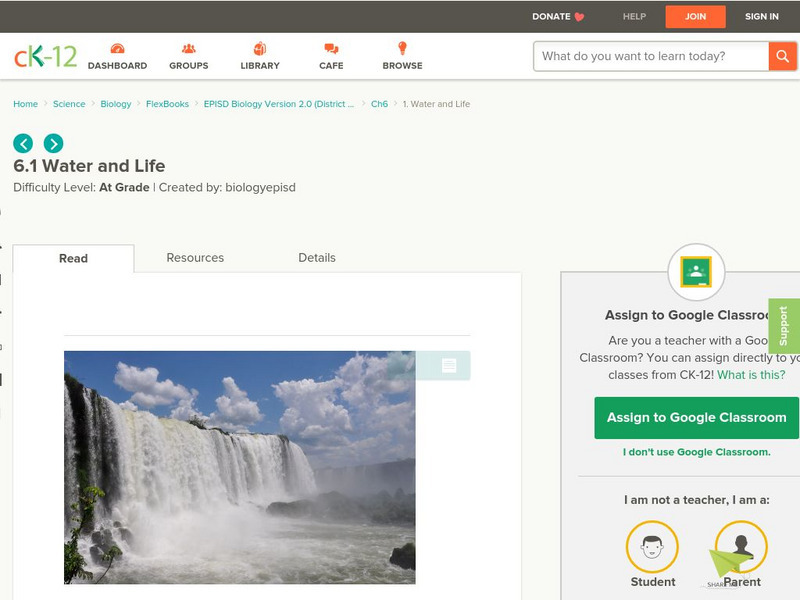
Ck 12: Water and Life
[Free Registration/Login may be required to access all resource tools.] The chemical and physical properties of water are examined in this lesson, including the role of hydrogen bonds in determining water's properties. The importance of...
CK-12 Foundation
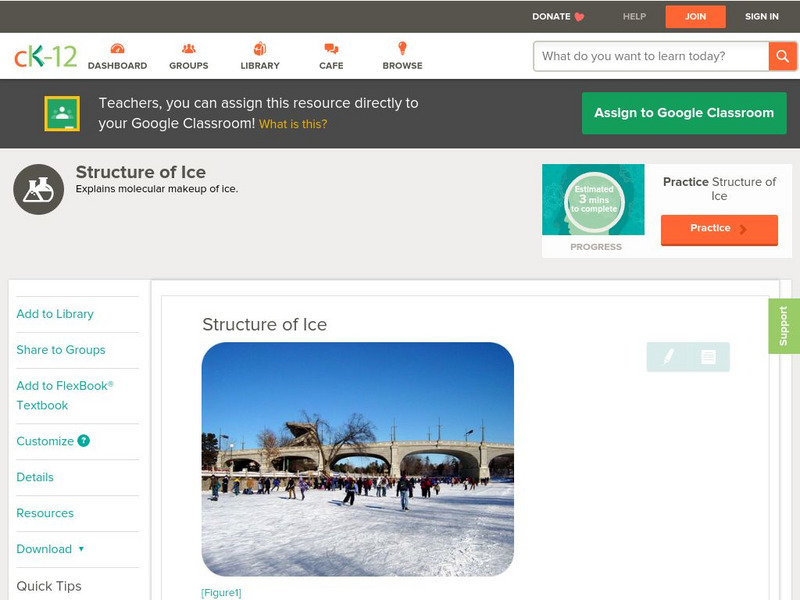
Ck 12: Chemistry: Structure of Ice
[Free Registration/Login may be required to access all resource tools.] Covers structure of ice and hydrogen bonding in ice.
Sophia Learning
Sophia: Characteristics of Chemical Bonds: Lesson 2
This lesson will present the basic properties and characteristics of chemical bonds. It is 2 of 4 in the series titled "Characteristics of Chemical Bonds."
Sophia Learning
Sophia: Characteristics of Chemical Bonds: Lesson 4
This lesson will present the basic properties and characteristics of chemical bonds. It is 4 of 4 in the series titled "Characteristics of Chemical Bonds."
Sophia Learning
Sophia: Molecular Structure of Water: Lesson 2
This lesson will introduce the molecular structure of water, including the Hydrogen atoms, Oxygen atoms, and types of bonds. It is 2 of 4 in the series titled "Molecular Structure of Water."
Sophia Learning
Sophia: Molecular Structure of Water: Lesson 3
This lesson will introduce the molecular structure of water, including the Hydrogen atoms, Oxygen atoms, and types of bonds. It is 3 of 4 in the series titled "Molecular Structure of Water."
University of Arizona
University of Arizona: Biochemistry
Problem sets, tutorials, and activities related to biochemistry.
Sophia Learning
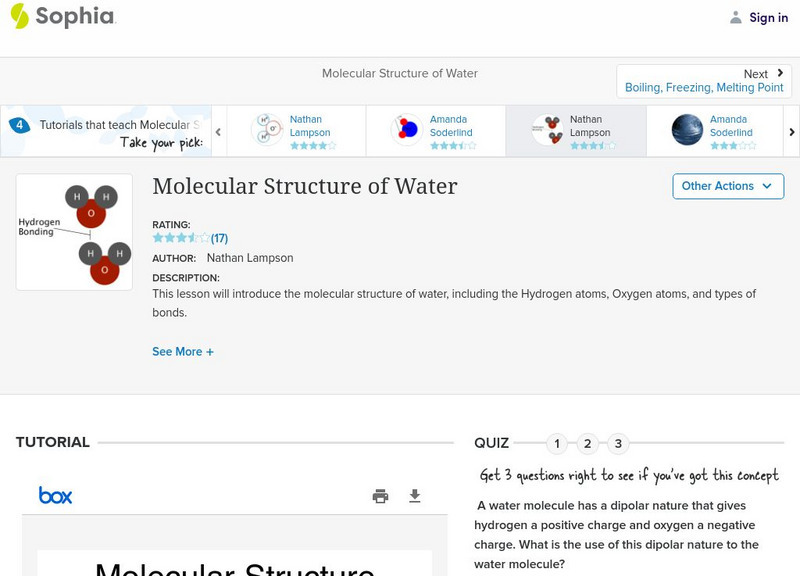
Sophia: Molecular Structure of Water
A brief introduction to the molecular structure of a water molecule, and the chemical bonding which gives it its unique properties.
University of Arizona
Ua: Chemistry Tutorial
This general tutorial begins with an explanation of the polarity of the water molecule and the effects this polarity has on the properties of water. Goes on to introduce organic molecules and has a thourough tutorial on the third page.
Khan Academy
Khan Academy: Biology: Water, Acids, and Bases: Cohesion and Adhesion of Water
This article defines and discusses cohesion and adhesion. Understand these two topics and their importance in hydrogen bonding.
Concord Consortium
Concord Consortium: Stem Resources: Intermolecular Attractions
Learn that boiling point, solubility, and DNA are affect by intermolecular forces in this module. Module includes lessons with questions and animations to explain London dispersion and dipole-dipole attractions. To conclude the lessons,...
Chem4kids
Chem4 Kids: Lithium
Here at Chem4Kids you can find some great information about the third element in the periodic table, lithium. Content focuses on lithium's electrons, where you can find lithium in nature, and how it bonds with other elements (or with...
University of Kentucky
University of Kentucky: Hydrogen Like Orbitals
Good site for viewing atomic orbitals. Click on an energy level and you get a simulated three dimensional image of the orbitals.
University of Florida
Chem. 2041 Lecture Notes: The Forces Between Molecules
A discussion of the variety of forces which hold molecules together. The relative strengths of these forces for the various states of matter is discussed. The effect of such forces on the boiling points and other phase change...
Iowa State University
Iowa State University: Percent of Water in a Crystalline Solid Hydrate
Lab activity uses a computer simulation to determine the amount of water in a crystalline solid hydrate.
Iowa State University
Iowa State University: Empirical Formula of a Compound
Use this computer simulation to determine the amount of water in a crystalline solid hydrate.